Under the Surface: Revealing the Mechanisms Behind Eating Disorders
Samuel Jacobs
Illustrations by Emily Holtz
Eating disorders such as anorexia nervosa and bulimia nervosa are serious, life‑threatening medical conditions that are often misunderstood as mere ‘vanity disorders’ due to an excessive preoccupation with body shape and weight [1, 2]. The most widely known kind of anorexia nervosa is the restrictive subtype, characterized by the persistent restriction of food relative to what an individual’s body needs [2]. On the other hand, bulimia and the binge-purge subtype of anorexia nervosa (AN-BP) are characterized by recurrent binge-eating episodes accompanied by purging behaviors, including vomiting, fasting, excessive exercise, or misuse of laxatives and diuretics [2]. The cycle of bingeing and purging is a behavioral loop that briefly relieves emotional distress, but ultimately reinforces harmful eating patterns [3, 4]. Individuals with bulimia may eat large amounts of food over a short period and feel unable to stop [3]. These binges are often followed by intense guilt or discomfort that compels the individual to engage in purging behaviors aimed at preventing weight gain [3, 5]. Patterns of restriction in anorexia nervosa and binge-purge in bulimia/AN-BP can both cause distinct structural alterations within the brain [6]. Specifically, physical changes can occur within neural circuits, the pathways between different brain regions [6]. Through the modification of neural circuits that underlie eating habits, behaviors can become permanently altered, contributing to the dangerous long-term nature of eating disorders [6]. Even after recovery, many individuals continue to experience residual symptoms, rather than a definitive return to a pre-disorder baseline [7]. Due to this, eating disorders have high relapse rates, and individuals in recovery remain vulnerable to returning to destructive behaviors [8, 9]. As a result, they could suffer from the long-lasting psychological and physical effects of these disorders for the rest of their lives [10, 11, 12].
Habit Over Health: The Mind-Body Disconnect
Individuals with bulimia have immense difficulty shifting their thinking away from binge-purge behaviors, even when these actions no longer provide a sense of reward or satisfaction [13]. In fact, these behaviors can persist despite the unpleasant consequences, ranging from emotional distress to dental decay [3, 14]. The brain relies more on ingrained harmful behaviors than flexible responses, such as recognizing hunger and fullness cues, eating regularly, or seeking emotional support [15]. The flexible responses require conscious reflection rather than thoughtless reaction [15]. Over time, repeated responses strengthen the specific neural connections responsible for these behavioral reactions, creating a mental ‘shortcut’ that makes binge-purge routines habitual and difficult to interrupt [15]. So, when feelings of shame or loss of control hit after bingeing, a person might automatically purge as a way to ‘undo’ the experience, even if they no longer believe it actually helps [3, 13]. If purging is not an option, individuals may struggle to figure out what to do next, since the brain pathways that normally help them move from emotional distress to flexible problem-solving are not communicating efficiently [15].
While bulimia is driven by compulsive reward-seeking through binge-purge behaviors, anorexia nervosa is driven by reward-seeking through rigid control and avoidance of foods [16, 17]. However, both share a fundamental disruption in the neural circuits underlying reward learning and habit formation [16, 18]. Similar to bulimia, the reward system of an individual with anorexia nervosa becomes less responsive to ordinary pleasures and more attuned to the sense of control that comes from restriction and the pleasure created from this control [19]. For example, a person with anorexia nervosa may avoid high-calorie and high-fat foods or adhere to strict meal rituals [17, 20]. The more a person restricts their food intake, the more distressing normal eating patterns can feel, whereas avoiding food can feel calming or purposeful [19, 21]. Once a particular behavior has been reinforced, such as food restriction, individuals with anorexia nervosa tend to persist in that pattern even when it starts causing life-threatening health effects like heart and blood-vessel complications or bone deterioration [21, 22, 23, 24]. This inflexibility is a characteristic of the restrictive and ritualized nature of eating behaviors in anorexia nervosa [19].
What is the Reward Driving Restriction? Dopamine Function in Anorexia Nervosa
In a healthy brain, dopamine serves as a chemical messenger that signals which experiences are rewarding, thereby motivating repeated behaviors [13]. The brain’s reward circuit relies on dopamine as a central component that guides decision-making and reinforces learning [25]. When a behavior, such as maintaining a daily calorie deficit, yields a more positive result than expected — for example, alleviating discomfort or expediting weight loss — dopamine levels increase, leading to behavior reinforcement [26]. Conversely, when a behavior, such as unintentionally overeating, turns out worse than expected and prompts feelings of discomfort, dopamine levels drop, teaching the brain to avoid this behavior in the future [13, 25]. A ‘two-stage’ model can be used to describe dopamine’s integral role in reinforcing anorexia nervosa [27]. First, dopamine activity may increase in response to dieting and excessive exercise, producing a sense of control and success that reinforces food restriction [27]. In the second stage, prolonged starvation lowers overall brain dopamine levels, making the dopamine receptors hypersensitive [27]. Consequently, a normal burst of dopamine from regular eating can feel abnormally intense, triggering an anxiety response instead of a pleasurable one [13, 27]. This heightened dopamine sensitivity in anorexia nervosa illustrates why normal eating can feel uncomfortable or anxiety-provoking, helping explain how restriction becomes reinforced even though it is ultimately harmful [26]. In this model, dopamine not only contributes to the onset of anorexia nervosa but also sustains the duration of the disorder by transforming short-term relief into unhealthy routines [27]. Additionally, binge-purge behaviors in bulimia may also engage dopamine signaling in a similar pattern to anorexia nervosa [28]. In bulimia, habit and reward-related brain circuits are dysregulated in a manner that promotes impulsive, reward-seeking behavior like bingeing, associated with decreased dopamine receptor binding [13]. Bulimia appears to be linked with lower dopamine sensitivity, so larger amounts of food are often needed to generate a noticeable sense of reward or satisfaction [26]. Thus, both disorders exhibit maladaptive patterns in the same underlying reward circuitry [30].
Veering Off Course: Misdirection in the Brain
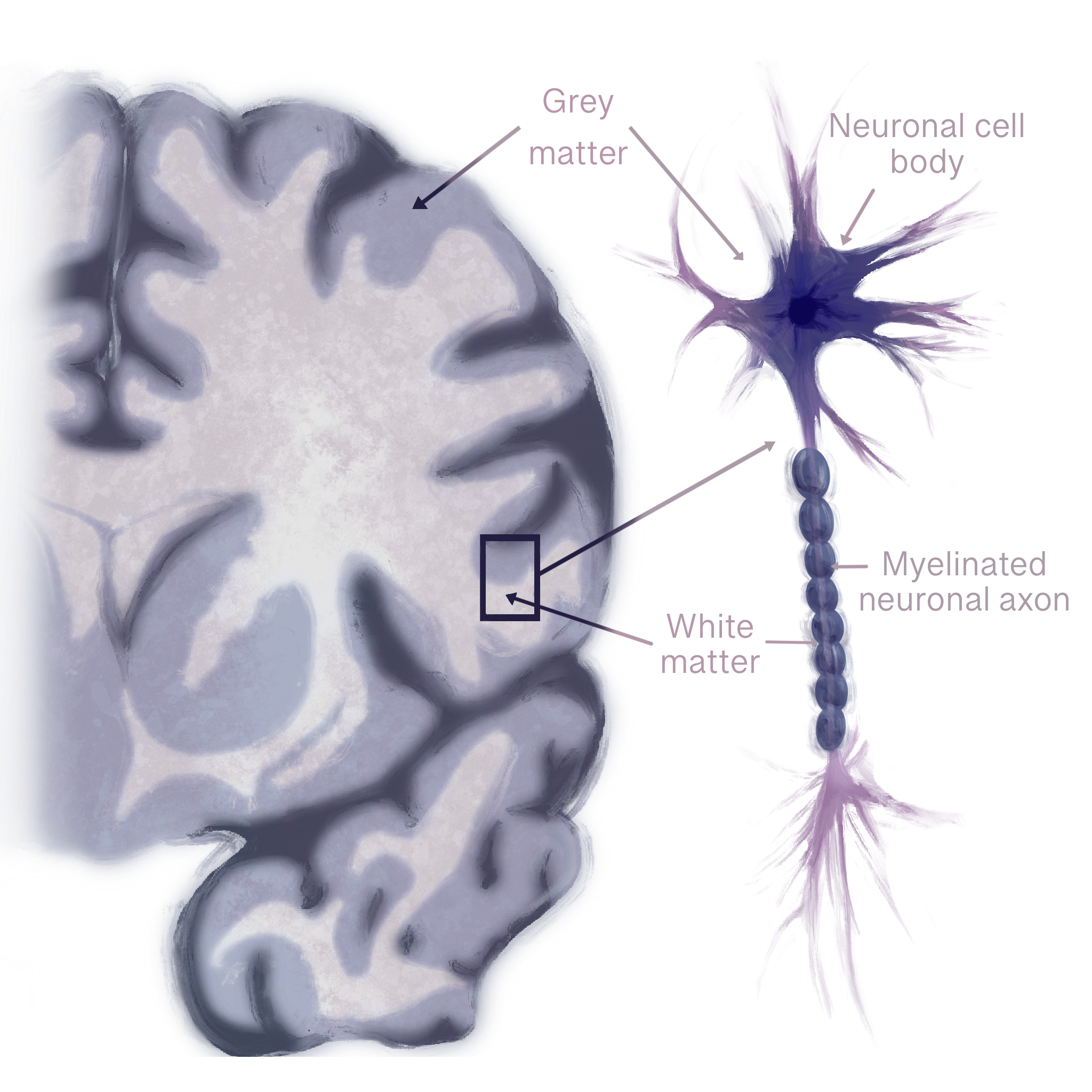
Eating disorders leave their mark not only on behavior but within the brain itself, where structural changes leave a lasting neurobiological impact [29, 30, 31]. The brain is composed of two main types of tissue: gray matter and white matter [32, 33]. Gray matter contains neurons that handle thinking and processing, while white matter consists of bundles of axons — long, slender projections of neurons that conduct electrical impulses away from the cell body — that are coated in an insulating fatty sheath, fostering quick communication between distant brain regions [32, 34, 35]. In anorexia nervosa, the white matter begins to thin due to malnutrition, which reduces coordination between areas that regulate reward, emotion, and self-perception [36, 37]. Think of white matter as a network of highways connecting different cities: when the roads are well-maintained, traffic flows smoothly, and messages are delivered efficiently to their intended destinations. Thinning of axons in these pathways is similar to potholes, lane closures, or damaged bridges on the highways — signals slow down, get misdirected, or fail to reach their destination, which can disrupt coordination between brain regions that regulate reward, emotions, and self-perception [37]. In the brain, these disruptions in physical wiring and coordinated regional activity represent a breakdown in overall connectivity [6]. People with anorexia nervosa experience reduced connectivity, meaning weaker communication between brain circuits like the default mode network, which is involved in self-focused thinking, and the salience network, which helps the brain detect important emotional or bodily signals like hunger or fullness [37]. Furthermore, loss of white matter fibers has been found in pathways connecting emotional and decision-making centers of the brain [37]. Reliance on rigid, well-worn habits over flexible decision-making can lead to sudden spikes of fear or distress around eating, making even small changes — like adding one new food to a meal — feel impossible [17]. Some white matter abnormalities improve during long-term weight restoration, while others persist and are associated with illness duration [18]. A longer illness duration predicts enduring white matter deficits, suggesting that the longer anorexia nervosa remains untreated, the more persistent these changes become [38]. Over time, structural disruptions may deepen and become harder to reverse, making early intervention critical to recovery [39].
In contrast, bulimia is characterized by abnormalities in brain regions that support habit formation and reward regulation [18, 38]. Disrupted communication within those regions can harm reward evaluation abilities and behavioral control [38]. Structural disturbances may contribute to the compulsive nature of binge-purge cycles and the difficulty of disengaging from them [40]. Moreover, bulimia is linked to structural alterations in brain regions that regulate reward, self-control, and emotional processing [18]. Abnormalities include reduced gray-matter volume or altered connectivity in key areas involved in evaluating rewards and regulating internal bodily sensations like hunger, fullness, and taste [18, 41]. Changes in underlying brain structure may reinforce maladaptive patterns of eating behavior and make it harder to resist urges to binge or purge [18]. Taken together, both anorexia nervosa and bulimia are marked by structural and connectivity disturbances that reinforce maladaptive behaviors; anorexia nervosa shows disrupted communication between self-perception and control networks, while bulimia shows dysfunctional integration within reward and habit systems [18, 23, 38].
Crumbling Connectivity and Shifts in Structure in Bulimia
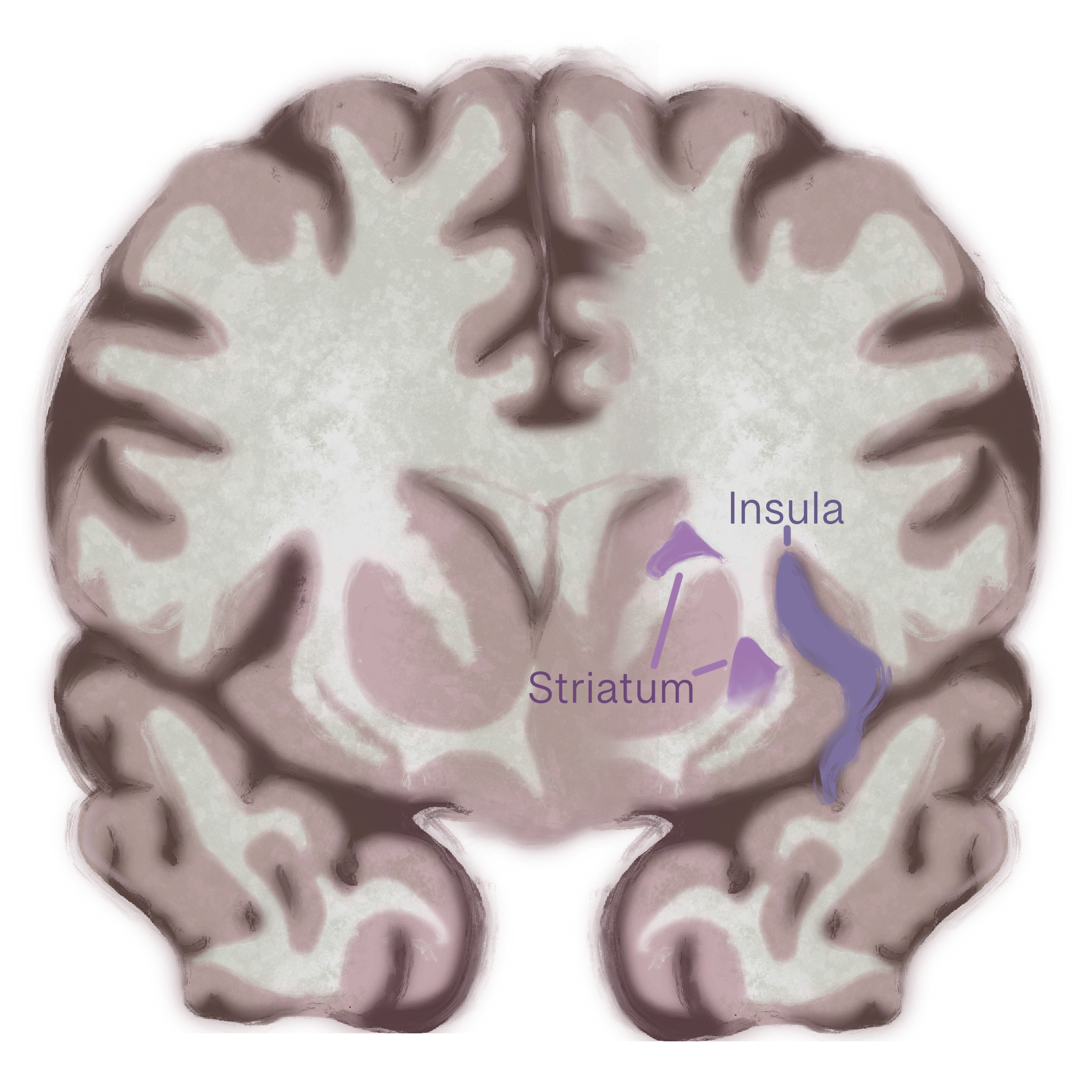
Structural changes in a brain region called the insula may drive binge-purge behavior in bulimia. Similar to anorexia nervosa, bulimia also affects the insula [13]. People with bulimia have less gray matter in the front part of the insula. Reductions in gray matter may indicate a decrease in healthy neurons in a given area, and that the insula does not function as efficiently when integrating emotions with physical urges or sensations in bulimia. Weakened integration could make it harder to regulate powerful urges to binge or purge, since the brain struggles to align emotional experiences with body signals. Disrupted activity in different subregions of the insula also suggests that cognition-emotion coordination is impaired, contributing to the impulsive, emotionally driven eating behaviors seen in bulimia. These findings highlight the insula’s key role in linking emotional states with bodily urges, showing how its dysfunction may fuel the binge-purge cycle [13]. Beyond structural changes in the insula, bulimia also disrupts communication between brain regions, particularly within circuits that link motivation, habits, and decision-making [38]. A key structure in this process is the striatum, a deep-brain region involved in learning what feels rewarding and shaping habitual behaviors [38]. In people with bulimia, the striatum shows abnormal communication with other regions such as the prefrontal cortex, thalamus, and sensorimotor areas — regions responsible for planning, emotional regulation, and body awareness. A miscommunication appears to disrupt the balance between the brain’s reward and control systems, which may underlie the recurring binge–purge cycle. When these systems fail to coordinate properly, urges to binge or purge can override rational decision-making, making the behavior feel automatic and difficult to stop [38].
Large-scale brain region networks that coordinate attention, emotion regulation, and self-control also appear to function differently in bulimia [42]. These networks, such as the fronto-parietal network (FPN) and the cingulo-opercular network (CON), are like the brain’s ‘command centers’ that help people stay focused on goals, manage impulses, and adapt their behavior [42]. The FPN supports flexible control and attention, while the CON helps maintain task focus and emotional stability over time [42, 43]. In bulimia, these large-scale networks show decreased connectivity, indicating weaker functional communication within and between them [42]. The degree of this disruption is linked to the severity of a person’s symptoms: those with more pronounced connectivity issues tend to experience stronger binge–purge urges and more emotional instability [42]. These findings suggest that bulimia involves widespread disruptions not only in reward and control circuits, but also in the broader networks that govern attention, self-control, and emotional regulation [38, 42].
Muted Metabolites: Weak Signals and Strong Body Image Issues
Just as the brain’s wiring patterns can shape habits and emotions, its chemical composition also plays a crucial role in the development and persistence of eating disorders [13]. Chemicals known as metabolites are small molecules that help neurons produce energy, communicate effectively, and repair themselves [13]. When these chemical systems are disrupted, the brain may struggle to regulate hunger, reward, and emotional balance [13]. Reduced levels of metabolites in the insula may indicate that the neurons are functioning, but not at full capacity [41]. Weakened neural signaling in the insula may contribute to the disconnect that many people with anorexia nervosa experience from their own bodily cues, making hunger feel confusing or even anxiety-provoking rather than natural [41]. Lower levels of the metabolite N-acetylaspartate (NAA) are strongly linked to greater concern about weight and body image, and therefore, these chemical changes may reinforce obsessive self-focus and distorted body perception [41]. Importantly, these chemical changes may not be permanent. As individuals begin the recovery process, metabolite levels in some regions, such as the insula, begin to return to typical ranges [41]. At this point, the brain can regain chemical balance with appropriate treatment and nutrition, thereby promoting the restoration of hunger cues [44]. However, many individuals still report lingering difficulties with body image, suggesting that emotional and cognitive patterns often return to baseline more slowly than brain chemistry [41]. Recovery from anorexia nervosa is both physical and psychological, requiring time for both the brain and self-perception to return to baseline [41].
In bulimia and AN-BP, brain chemistry follows a somewhat different pattern from anorexia nervosa [45]. Both disorders involve abnormal activity in brain regions responsible for reward, self-control, and emotional awareness, but the underlying neurochemistry is not identical. People with AN-BP showed reduced levels of NAA and myo-inositol — chemicals that support healthy brain cell membranes and energy use — in the prefrontal cortex, a region involved in impulse regulation, and the occipital lobe, an area involved in visual information. This could mean that the brains of individuals with AN-BP are less efficient at regulating impulses and integrating visual information related to body image [45]. Conversely, people with bulimia do not show the same reductions in certain brain chemicals seen in anorexia nervosa, suggesting that their symptoms may arise from a different pattern of brain activity related to how rewards are processed, rather than from widespread reductions in brain efficiency [45]. Together, these findings suggest that while both anorexia nervosa and bulimia affect overlapping networks in the brain, each disorder uniquely disrupts the brain chemistry; therefore, effective treatment might need to target not just behavior, but also the specific neurochemical imbalances underlying each condition [41, 45].
Mind Games: Rewiring Maladaptive Circuits
Eating disorders can be treated through pharmacological, psychological, or neurological methods [46, 47, 48]. Anti-depressant and anti-anxiety medications treat anorexia nervosa and bulimia by reducing co-occurring depression or anxiety that reinforce restrictive behaviors, and are often used in tandem with psychotherapy for further symptom reduction [28, 46, 49]. Cognitive Behavioral Therapy for Eating Disorders (CBT-ED) is the most widely supported psychological approach across eating disorder diagnoses [47]. CBT-ED targets the cognitive and behavioral mechanisms that sustain disordered eating — such as strict dieting, over-valuation of shape and weight, and maladaptive emotion regulation [47]. Cognitive Behavioral Therapy for anorexia nervosa (CBT-AN) helps individuals recognize and modify patterns that maintain restriction or obsessive focus on weight [50]. Patients who receive CBT-AN show greater weight gain and larger reductions in eating disorder symptoms compared to standard care [50]. Cognitive-training programs that aim to dissuade urges to binge-purge and strengthen inhibitory control are showing promise in reducing compulsive eating patterns in bulimia as well [16, 45]. CBT-ED protocols address the specific psychological and neurobehavioral mechanisms that maintain anorexia nervosa and bulimia, regardless of whether the presentation is restrictive or binge-purge [51].
Similarly, emerging therapies that directly target brain circuits offer another avenue for treating anorexia nervosa and bulimia [48, 52]. Transcranial direct current stimulation (tDCS) is a non-invasive technique that applies a mild electrical current to specific brain areas, aiming to improve cognitive control, emotional regulation, and food intake [49, 53, 54]. Depressive symptoms, which often co-occur with anorexia nervosa and bulimia, may be reduced by tDCS [48]. While still experimental, these interventions aim to strengthen the brain circuits that are disrupted in anorexia nervosa and bulimia [16, 48, 52]. In anorexia nervosa, tDCS helps patients regain more flexible responses to food and emotional cues, similar to findings reported in bulimia [48, 52]. After only a few sessions of tDCS, individuals with bulimia reported suppression of binge-purge urges and increases in self-regulatory control [52]. Multi-session trials would be necessary to validate this approach as a clinical treatment method for bulimia; however, the findings to date have been positive [52]. By targeting the neural underpinnings of the disorder, neurofeedback and brain-stimulation techniques could eventually complement traditional therapy and behavioral interventions.
Rebuilding: The Road to Rehabilitation
While both anorexia nervosa and bulimia share core features of disrupted eating behaviors, body-image disturbance, and altered brain processes, the neurobiological pathways that sustain each disorder diverge in meaningful ways [13, 19]. Anorexia nervosa is characterized by extreme food restriction, heightened dopamine sensitivity, rigid habit formation, and structural white-matter and connectivity changes that reflect a brain entrenched in avoidance and control [25, 27, 37]. Bulimia and AN-BP, however, are marked by binge-purge cycles, reduced dopamine responsiveness, impulsive reward-seeking, and alterations in habit and reward-based circuitry [13, 28, 30]. Structural and functional brain alterations in eating disorders continue even after physical recovery, indicating persistent neurobiological vulnerability even after symptomatic improvement [29, 30]. Therefore, recovery not only involves returning to normalized eating behaviors but also restoring healthier brain circuit function and encouraging flexible behavioral patterns [41, 44]. Treatment should be tailored to these neural profiles: for bulimia, interventions may focus on disrupting maladaptive habit loops and retraining reward sensitivity, while for anorexia nervosa, strategies may focus on targeting dopamine or metabolite-related pathways to rewire the brain’s rigid control systems toward adaptive regulation [16, 26, 27, 42]. Early intervention is critical because the longer pathological reward or habit patterns persist, the more entrenched and difficult they become to reverse [38, 39]. By viewing anorexia nervosa and bulimia not just as psychiatric disorders, but as disorders of neural circuitry and learned behavior, we gain a clearer framework for precise treatments and innovative therapeutic approaches [18, 52].
References
Aird, C. S., Reisinger, B. A., Webb, S. N., & Gleaves, D. H. (2025). Comparing social stigma of anorexia nervosa, bulimia nervosa, and binge-eating disorder: A quantitative experimental study. Journal of Eating Disorders, 13(1). https://doi.org/10.1186/s40337-025-01198-x
American Psychiatric Association. (2022). Feeding and eating disorders. In Diagnostic and Statistical Manual of Mental Disorders (5th ed., text rev.). https://doi.org/10.1176/appi.books.9780890425596
Rouhani, N., Grossman, C. D., Feusner, J., & Tusche, A. (2025). Eating disorder symptoms and emotional arousal modulate food biases during reward learning in females. Nature Communications, 16(1), 2938. https://doi.org/10.1038/s41467-025-57872-w
Serra, R., Di Nicolantonio, C., Di Febo, R., De Crescenzo, F., Vanderlinden, J., Vrieze, E., Bruffaerts, R., Loriedo, C., Pasquini, M., & Tarsitani, L. (2022). The transition from restrictive anorexia nervosa to binging and purging: A systematic review and meta-analysis. Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity, 27(3), 857–865. https://doi.org/10.1007/s40519-021-01226-0
Wilson, K., & Kagabo, R. (2024). Bulimia nervosa and treatment-related disparities: A review. Frontiers in Psychology, 15. https://doi.org/10.3389/fpsyg.2024.1386347
Frank, G. K. W. (2019). Neuroimaging and eating disorders. Current Opinion in Psychiatry, 32(6), 478–483. https://doi.org/10.1097/YCO.0000000000000544
Adame, A. L., Pierce, E., Jimenez, A., Shelby, T., & Parks, D. (2024). How Does Self-Identity Change in Eating Disorder Recovery? Journal of Humanistic Psychology, 00221678241255264. https://doi.org/10.1177/00221678241255264
Miskovic-Wheatley, J., Bryant, E., Ong, S. H., Vatter, S., Le, A., Aouad, P., Barakat, S., Boakes, R., Brennan, L., Bryant, E., Byrne, S., Caldwell, B., Calvert, S., Carroll, B., Castle, D., Caterson, I., Chelius, B., Chiem, L., Clarke, S., … National Eating Disorder Research Consortium. (2023). Eating disorder outcomes: Findings from a rapid review of over a decade of research. Journal of Eating Disorders, 11(1), 85. https://doi.org/10.1186/s40337-023-00801-3
Khalsa, S. S., Portnoff, L. C., McCurdy-McKinnon, D., & Feusner, J. D. (2017). What happens after treatment? A systematic review of relapse, remission, and recovery in anorexia nervosa. Journal of Eating Disorders, 5(1), 20. https://doi.org/10.1186/s40337-017-0145-3
Bardone-Cone, A. M., Hunt, R. A., & Watson, H. J. (2018). An Overview of Conceptualizations of Eating Disorder Recovery, Recent Findings, and Future Directions. Current Psychiatry Reports, 20(9), 79. https://doi.org/10.1007/s11920-018-0932-9
McDonald, S., Williams, A. J., Barr, P., McNamara, N., & Marriott, M. (2021). Service user and eating disorder therapist views on anorexia nervosa recovery criteria. Psychology and Psychotherapy: Theory, Research and Practice, 94(3), 721–736. https://doi.org/10.1111/papt.12340
Kenny, T. E., Trottier, K., & Lewis, S. P. (2022). Lived experience perspectives on a definition of eating disorder recovery in a sample of predominantly white women: A mixed-method study. Journal of Eating Disorders, 10(1), 149. https://doi.org/10.1186/s40337-022-00670-2
Li, W., Wang, Y., Wang, J., Wang, M., Liu, J., Chen, Q., Yang, Z., Li, Z., Wu, G., Wang, Z., Zhang, P., & Tang, L. (2024). Bulimia nervosa selectively reshapes the structure and intrinsic function of anterior insula subregions associated with cognition-emotion integration. Journal of Affective Disorders, 362, 529–535. https://doi.org/10.1016/j.jad.2024.07.051
Nijakowski, K., Jankowski, J., Gruszczyński, D., & Surdacka, A. (2023). Eating Disorders and Dental Erosion: A Systematic Review. Journal of Clinical Medicine, 12(19), 6161. https://doi.org/10.3390/jcm12196161
Dougherty, E. N., Wildes, J. E., & Haedt-Matt, A. A. (2024). The role of habit in maintaining binge/purge behaviors: An ecological momentary assessment study. International Journal of Eating Disorders, 57(5), 1160–1171. https://doi.org/10.1002/eat.24070
Berner, L. A., Fiore, V. G., Chen, J. Y., Krueger, A., Kaye, W. H., Viranda, T., & de Wit, S. (2023). Impaired belief updating and devaluation in adult women with bulimia nervosa. Translational Psychiatry, 13(1), 2. https://doi.org/10.1038/s41398-022-02257-6
Conceição, I. S. R., Garcia-Burgos, D., de Macêdo, P. F. C., Nepomuceno, C. M. M., Pereira, E. M., Cunha, C. de M., Ribeiro, C. D. F., & de Santana, M. L. P. (2023). Habits and Persistent Food Restriction in Patients with Anorexia Nervosa: A Scoping Review. Behavioral Sciences, 13(11), 883. https://doi.org/10.3390/bs13110883
Berner, L. A., Wang, Z., Stefan, M., Lee, S., Huo, Z., Cyr, M., & Marsh, R. (2019). Subcortical shape abnormalities in bulimia nervosa. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(12), 1070–1079. https://doi.org/10.1016/j.bpsc.2018.12.011
Haynos, A. F., Anderson, L. M., Askew, A. J., Craske, M. G., & Peterson, C. B. (2021). Adapting a neuroscience-informed intervention to alter reward mechanisms of anorexia nervosa: A novel direction for future research. Journal of Eating Disorders, 9(1), 63. https://doi.org/10.1186/s40337-021-00417-5
Foerde, K., Schebendach, J. E., Davis, L., Daw, N., Walsh, B. T., Shohamy, D., & Steinglass, J. E. (2022). Restrictive eating across a spectrum from healthy to unhealthy: Behavioral and neural mechanisms. Psychological Medicine, 52(9), 1755–1764. https://doi.org/10.1017/S0033291720003542
Wierenga, C. E., Reilly, E., Bischoff-Grethe, A., Kaye, W. H., & Brown, G. G. (2022). Altered Reinforcement Learning from Reward and Punishment in Anorexia Nervosa: Evidence from Computational Modeling. Journal of the International Neuropsychological Society, 28(10), 1003–1015. https://doi.org/10.1017/S1355617721001326
Springall, G. A. C., Caughey, M., Zannino, D., Kyprianou, K., Mynard, J. P., Rudolph, S., Cheong, J., Yeo, M., & Cheung, M. M. H. (2023). Long-term cardiovascular consequences of adolescent anorexia nervosa. Pediatric Research, 94(4), 1457–1464. https://doi.org/10.1038/s41390-023-02521-5
Friars, D., Walsh, O., & McNicholas, F. (2023). Assessment and management of cardiovascular complications in eating disorders. Journal of Eating Disorders, 11(1), 13. https://doi.org/10.1186/s40337-022-00724-5
Gibson, D., Filan, Z., Westmoreland, P., & Mehler, P. S. (2024). Loss of Bone Density in Patients with Anorexia Nervosa Food That Alone Will Not Cure. Nutrients, 16(21), 3593. https://doi.org/10.3390/nu16213593
Hart, G., Burton, T. J., & Balleine, B. W. (2024). What Role Does Striatal Dopamine Play in Goal-directed Action? Neuroscience, 546, 20–32. https://doi.org/10.1016/j.neuroscience.2024.03.020
Lopez, G. C., & Lerner, T. N. (2025). How dopamine enables learning from aversion. Current Opinion in Behavioral Sciences, 61, 101476. https://doi.org/10.1016/j.cobeha.2024.101476
Beeler, J. A., & Burghardt, N. S. (2022). The rise and fall of dopamine: A two-stage model of the development and entrenchment of anorexia nervosa. Frontiers in Psychiatry, 12, 799548. https://doi.org/10.3389/fpsyt.2021.799548
Yu, Y., Miller, R., & Groth, S. W. (2022). A literature review of dopamine in binge eating. Journal of Eating Disorders, 10(1), 11. https://doi.org/10.1186/s40337-022-00531-y
Hagan, K., Lloyd, E. C., & Gorrell, S. (n.d.). Annual Research Review: Neural mechanisms of eating disorders in youth – from current theory and findings to future directions. Journal of Child Psychology and Psychiatry, n/a(n/a). https://doi.org/10.1111/jcpp.70029
Steinglass, J. E., & Walsh, B. T. (2016). Neurobiological model of the persistence of anorexia nervosa. Journal of Eating Disorders, 4, 19. https://doi.org/10.1186/s40337-016-0106-2
Su, T., Gong, J., Tang, G., Qiu, S., Chen, P., Chen, G., Wang, J., Huang, L., & Wang, Y. (2021). Structural and functional brain alterations in anorexia nervosa:A multimodal meta-analysis of neuroimaging studies. Human Brain Mapping, 42(15), 5154–5169. https://doi.org/10.1002/hbm.25602
Timmler, S., & Simons, M. (2019). Grey matter myelination. Glia, 67(11), 2063–2070. https://doi.org/10.1002/glia.23614
Sampaio-Baptista, C., & Johansen-Berg, H. (2017). White Matter Plasticity in the Adult Brain. Neuron, 96(6), 1239–1251. https://doi.org/10.1016/j.neuron.2017.11.026
Alcami, P., & El Hady, A. (2019). Axonal Computations. Frontiers in Cellular Neuroscience, 13. https://doi.org/10.3389/fncel.2019.00413
Chorghay, Z., Káradóttir, R. T., & Ruthazer, E. S. (2018). White Matter Plasticity Keeps the Brain in Tune: Axons Conduct While Glia Wrap. Frontiers in Cellular Neuroscience, 12. https://doi.org/10.3389/fncel.2018.00428
Krasner, H., Ong, C. V., Hewitt, P., & Vida, T. A. (2025). From Stress to Synapse: The Neuronal Atrophy Pathway to Mood Dysregulation. International Journal of Molecular Sciences, 26(7), 3219. https://doi.org/10.3390/ijms26073219
de la Cruz, F., Schumann, A., Rieger, K., Giuliano, M. D., & Bär, K. J. (2023). Fibre-specific white matter changes in anorexia nervosa. Psychiatry Research: Neuroimaging, 336, 111736. https://doi.org/10.1016/j.pscychresns.2023.111736
Wang, L., Bi, K., Song, Z., Zhang, Z., Li, K., Kong, Q. M., Li, X. N., Lu, Q., & Si, T. M. (2020). Disturbed resting-state whole-brain functional connectivity of striatal subregions in bulimia nervosa. The International Journal of Neuropsychopharmacology, 23(6), 356–365. https://doi.org/10.1093/ijnp/pyaa023
Geisler, D., Roessner, V., Biemann, R., Marxen, M., & Ehrlich, S. (2019). Dynamic changes in white matter microstructure in anorexia nervosa: Findings from a longitudinal study. Psychological Medicine, 49(9), 1555–1564. https://doi.org/10.1017/S003329171800212X
Donnelly, B., Touyz, S., Hay, P., Burton, A., Russell, J., & Caterson, I. (2018). Neuroimaging in bulimia nervosa and binge eating disorder: A systematic review. Journal of Eating Disorders, 6(1), 3. https://doi.org/10.1186/s40337-018-0187-1
Maier, S., Nickel, K., Perlov, E., Kukies, A., Zeeck, A., van Elst, L. T., Endres, D., Spieler, D., Holovics, L., Hartmann, A., Dacko, M., Lange, T., & Joos, A. (2020). Insular cell integrity markers linked to weight concern in anorexia nervosa: An MR-spectroscopy study. Journal of Clinical Medicine, 9(5), 1292. https://doi.org/10.3390/jcm9051292
Lan, Z., Zhu, L. L., Wu, Y. K., Yang, J. J., Li, J. T., Zeng, Y. W., Li, K., Kong, Q. M., Su, Y. A., & Si, T. (2023). Aberrant modular segregation of brain networks in female patients with bulimia nervosa. The International Journal of Eating Disorders, 56(7), 1353–1364. https://doi.org/10.1002/eat.23939
D’Andrea, C. B., Laumann, T. O., Newbold, D. J., Nelson, S. M., Nielsen, A. N., Chauvin, R., Marek, S., Greene, D. J., Dosenbach, N. U. F., & Gordon, E. M. (2023). Substructure of the Brain’s CINGULO-Opercular Network. https://doi.org/10.1101/2023.10.10.561772
Seidel, M., Markmann Jensen, S., Healy, D., Dureja, A., Watson, H. J., Holst, B., Bulik, C. M., & Sjögren, J. M. (2021). A systematic review and meta-analysis finds increased blood levels of all forms of ghrelin in both restricting and binge-eating/purging subtypes of anorexia nervosa. Nutrients, 13(2), 709. https://doi.org/10.3390/nu13020709
Westwater, M. L., Murley, A. G., Diederen, K. M. J., et al. (2022). Characterizing cerebral metabolite profiles in anorexia and bulimia nervosa and their associations with habitual behavior. Translational Psychiatry, 12, 103. https://doi.org/10.1038/s41398-022-01872-7
Chiu, H.-P., Huang, M.-W., Tsai, S.-Y., & Hsu, C.-Y. (2023). A retrospective study of pharmacological treatment in anorexia nervosa: 6-month and 12-month follow-up. BMC Psychiatry, 23(1), 126. https://doi.org/10.1186/s12888-023-04604-3
Agras, W. S., & Bohon, C. (2021). Cognitive Behavioral Therapy for the Eating Disorders. Annual Review of Clinical Psychology, 17(Volume 17, 2021), 417–438. https://doi.org/10.1146/annurev-clinpsy-081219-110907
Chmiel, J., Gladka, A., & Leszek, J. (2023). The Effect of Transcranial Direct Current Stimulation (tDCS) on Anorexia Nervosa: A Narrative Review. Nutrients, 15(20), 4455. https://doi.org/10.3390/nu15204455
Kirchberg, M. C., Pinson, C., & Frank, G. K. W. (2024). Pharmacotherapeutic strategies for the treatment of anorexia nervosa – novel targets to break a vicious cycle. Expert Opinion on Pharmacotherapy, 25(17), 2253–2265. https://doi.org/10.1080/14656566.2024.2424316
Nohara, N., Yamanaka, Y., Matsuoka, M., Yamazaki, T., Kawai, K., Takakura, S., Sudo, N., Ando, T., Matsuyama, Y., Byrne, S., Dalle Grave, R., Cooper, Z., & Yoshiuchi, K. (2023). A multi-center, randomized, parallel-group study to compare the efficacy of enhanced cognitive behavior therapy (CBT-E) with treatment as usual (TAU) for anorexia nervosa: Study protocol. BioPsychoSocial Medicine, 17(1), 20. https://doi.org/10.1186/s13030-023-00277-2
Murphy, R., Bailey-Straebler, S., Dalle Grave, R., Calugi, S., Osborne, E. L., & Cooper, Z. (2025). Evolving perspectives on CBT-E for eating disorders: Clarifying ten key points – misconceptions and communication gaps explored. The Cognitive Behaviour Therapist, 18. https://doi.org/10.1017/s1754470x25100299
Kekic, M., McClelland, J., Bartholdy, S., Boysen, E., Musiat, P., Dalton, B., Tiza, M., David, A. S., Campbell, I. C., & Schmidt, U. (2017). Single-Session Transcranial Direct Current Stimulation Temporarily Improves Symptoms, Mood, and Self-Regulatory Control in Bulimia Nervosa: A Randomised Controlled Trial. PLOS ONE, 12(1), e0167606. https://doi.org/10.1371/journal.pone.0167606
Phillipou, A., Kirkovski, M., Castle, D. J., Gurvich, C., Abel, L. A., Miles, S., & Rossell, S. L. (2019). High-definition transcranial direct current stimulation in anorexia nervosa: A pilot study. International Journal of Eating Disorders, 52(11), 1274–1280. https://doi.org/10.1002/eat.23146
Baumann, S., Mareš, T., Albrecht, J., Anders, M., Vochosková, K., Hill, M., Bulant, J., Yamamotová, A., Štastný, O., Novák, T., Holanová, P., Lambertová, A., & Papežová, H. (2021). Effects of Transcranial Direct Current Stimulation Treatment for Anorexia Nervosa. Frontiers in Psychiatry, 12. https://doi.org/10.3389/fpsyt.2021.717255