Undercooked and Overlooked: When Worms Infest the Brain
Jack Matter
Illustrations by Lola Yost
An ordinary decision, such as what you order for lunch, could leave you vulnerable to parasitic tapeworms, unwanted guests that can wreak havoc on your body [1, 2, 3]. You may think the worst-case scenario from consuming undercooked or contaminated foods is a tapeworm in your gut, but they can actually invade your brain tissue as well [4]. Adult tapeworms use spiny hooks and suckers to latch on to the intestinal wall of a human host, siphoning nutrients from them [5]. As the worm grows, it sheds new eggs into the host’s feces. If another person ingests these eggs, the complex life cycle of the tapeworm begins to unfold [4]. The eggs can release tapeworm larvae that enter the host’s bloodstream and reach their most sensitive tissues, including the brain and spinal cord [4]. Neurocysticercosis (NCC) is a parasitic infection of the brain and spinal cord that begins when individuals accidentally ingest the eggs of tapeworms from the Taenia family, most often Taenia solium [1, 2, 6]. Some of the defining symptoms of NCC include seizures, headaches, and increased pressure on the brain [3]. In fact, NCC is a leading cause of preventable epilepsy in many parts of the world [3]. Effectively addressing NCC in areas where it is common requires a holistic approach that integrates individually tailored treatment plans, improved diagnostic tools, and robust public health investments [3, 6].
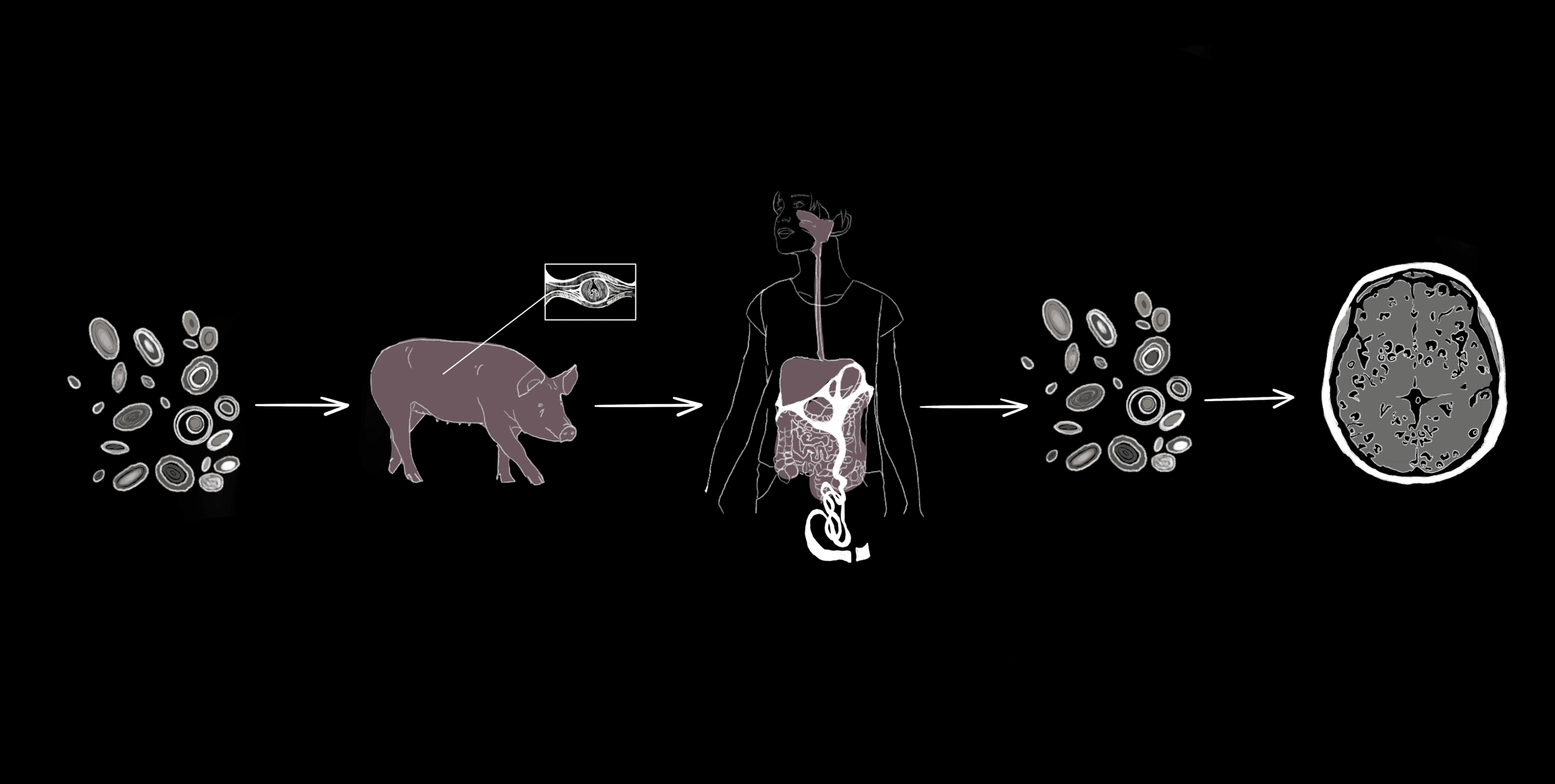
From Pork to Brain: Transmission of NCC
On the outskirts of a farm, sewage runoff contaminated with tiny T. solium tapeworm eggs has seeped into the soil [3]. A pig sniffs around in the dirt, scavenging for food, and coincidentally ingests the eggs [3]. The eggs are the first stage of the tapeworm’s complex life cycle, which includes several larval phases before fully mature adult worms develop [7]. After the pig ingests the eggs that protect the tapeworm embryo, the larvae emerge from the egg to start the next phase of their life cycle in the pig’s intestines [3, 7]. The larvae burrow into the pig’s muscles and form protective fluid-filled sacs around themselves called cysticerci [3, 6]. Eventually, a farmer slaughters the cysticerci-infected pig and sells it to a food vendor. The vendor sears the pork kebab on a grill but fails to cook it all the way, leaving live larvae inside the cysticerci [8]. A traveler stopping for dinner buys the undercooked meat and unknowingly ingests the cysticerci containing tapeworm larvae [8]. Over the next several weeks, an adult tapeworm can emerge from each ingested cysticercus, mature in the traveler’s intestine, and produce more eggs, which are shed into the traveler’s feces [2].
The traveler continues on their journey and develops mild abdominal pain, unaware of their new guest [3]. They do not wash their hands thoroughly after using the restroom, and traces of tapeworm eggs are left on doorknobs, restaurant tables, and even directly on food shared with others in their hostel [3]. The hostel guests who come into contact with the shared surfaces ingest the eggs, unknowingly putting themselves at risk for a dangerous infection. Unlike the initial traveler who ingested cysticerci, a hostel guest named Paul ingests tapeworm eggs [4]. The larvae of the tapeworm T. solium hatch from the eggs and invade Paul’s body [9]. The larvae then pass through Paul’s intestinal wall and enter the bloodstream, giving them access to muscle, the spinal cord, and brain tissues [2]. The larvae can embed in these tissues and develop into cysticerci, resulting in the infectious disease cysticercosis [2, 3, 10].
NCC, a specific type of cysticercosis, occurs when the hatched T. solium tapeworm larvae migrate through the blood to embed within the human brain or spinal cord, the organs that make up the central nervous system (CNS) [2]. The cells in the immune system can usually detect and respond to foreign threats like NCC in the body with the help of markers called antigens that are present on the outside of most cells [11]. Antigens trigger certain immune cells to produce corresponding molecules called antibodies. When antibodies bind to their specific antigens, they flag these foreign threats to activate immune responses that can ultimately kill an infection [11]. In the case of Paul’s parasitic infection, the immune system should react with an inflammatory response during which chemical signals are released to attract immune cells to the infection site [12]. Recruited immune cells would then release chemicals that can either destroy the parasite or contribute to further inflammation [12, 13]. Consequently, to successfully survive in the CNS, cysticerci must avoid being detected by the immune system [13].
While the immune system is designed to react swiftly to harmful invaders, cysticerci can suppress the host’s inflammatory responses, allowing them to go undetected by the immune system for extended periods of time [13, 14]. The absence of a robust inflammatory response may allow an individual with NCC to live completely symptom-free for many months [6, 13]. Cysticerci may use several strategies to avoid immune detection [13]. Say a bank robbery is underway; in this example, the robbers are the cysticerci, and the bank teller is an immune cell. The bank teller would normally spot the robbers and immediately alert authorities. In the case of a parasite, immune cells would signal other immune cells to destroy the cysticerci. The robbers anticipate this, however, and create a diversion or tie up the teller to prevent the alarm from ever going off. Similarly, cysticerci release antigens that draw immune cells away from them or prevent immune signaling cells from alerting the immune killer cells. As a result, the immune system remains unaware of the threat. Another strategy the bank robbers may use is a disguise, like dressing up as bank employees. The cysticerci can mask themselves from the immune system by covering their outer layer with the host’s own antibodies, tricking immune cells into thinking that the cysticerci are a part of the body and belong there [13]. Using these strategies, cysticerci can persist inside a host for a long time without causing any symptoms [6, 13].
Infiltration, Inflammation, and the Immune Attack
After months or even years, cysticerci can break down for unknown reasons, losing their ability to evade the immune system and subsequently activating an inflammatory response from the host [6, 9, 13]. Paul’s more serious symptoms, such as intense headaches and seizures, started with the activation of the inflammatory response [3, 9, 14]. For many individuals, the severity of these symptoms is typically associated with the intensity of the immune response in the brain [4, 9]. Following the immune system response, the cells that comprise the blood-brain barrier — a protective layer of cells that selectively prevent substances from entering the brain — also experience disruptions to their function [9, 13]. When the blood-brain barrier is compromised, immune cells usually excluded from the brain are allowed in and can then identify and attack broken-down cysticerci directly [9, 13]. Furthermore, immune attacks on cysticerci can be both beneficial and harmful [13]. On one hand, the cysticerci are being destroyed and the larvae are being killed, removing live parasites from the body. On the other hand, the destruction of cysticerci can lead to dangerous levels of immune inflammation in the brain that can severely damage surrounding tissues and cause more extreme symptoms [13].
A variety of NCC symptoms can arise depending on where the cysticerci are physically located within the CNS [6]. Broadly, there are two main types of NCC in the brain [10]. The most common type of NCC occurs when cysticerci form in the brain tissue itself — made up of nerves and supportive cells — called parenchymal NCC. Another type of NCC develops when cysticerci form in the spaces between brain tissues, including fluid-filled spaces and blood vessels, known as extraparenchymal NCC. Each type of NCC affects different brain regions and causes varying symptoms [10]. In parenchymal NCC, physical pressure or vessel blockages from the cysticerci and inflammation from the immune response may damage brain tissue, causing symptoms including headaches, vomiting, and even numbness in specific areas like the face [10, 15]. Paul, our hostel guest, suffers from the less common type, extraparenchymal NCC, which can cause similar symptoms to parenchymal NCC but may also lead to other fatal complications, such as stroke or increased pressure within the skull [15, 16]. Increased skull pressure is particularly dangerous because it can squeeze the brain and its blood vessels, ultimately leading to tissue damage, which can be permanent and even deadly [17].
Both types of NCC can also trigger seizures and lead to the development of epilepsy, an umbrella term for a disorder in which brain abnormalities cause recurring seizures [10, 18]. NCC is a leading cause of epilepsy in areas of the world where the parasitic infection is most prevalent [18]. The exact reason why NCC causes seizures is poorly understood, but it is believed that the inflammatory response plays a significant role [19]. Additionally, the likelihood of developing epilepsy from infectious and other types of lesions — areas of damaged tissue — depends on their location and size in certain parts of the brain [10, 20, 21]. Cysticerci are considered a type of lesion that contributes to scarring and damage to surrounding nerve cells, which may lead to epilepsy, as seen in Paul’s case [10]. Repeated seizures can continue even after the cysticerci are destroyed and become hardened, inactive lesions called calcified cysts [10, 19]. As many as half of individuals with NCC-associated epilepsy and calcified cysts experience chronic seizures ranging from months to years after cysticerci become calcified, further complicating our understanding of how cysticerci contribute to epilepsy. One possible explanation is that brain tissue may swell and scar around these calcified cysts, which is often noticed in individuals with seizures [10, 19]. However, it is unclear if the swelling and scarring cause the seizures or if seizures lead to these changes [19]. Another idea is that the calcified cysts might slowly release large amounts of calcium into the brain. Calcium is an important chemical messenger in many normal signaling processes, but too much calcium can harm brain cells. If nearby brain cells sustain damage or begin to die from excessive calcium exposure, they could release increased amounts of other chemical signaling molecules associated with seizures. Surgical removal of calcified cysts decreased the occurrence of seizures in a limited number of individuals, but the risk of brain surgery may not outweigh the theoretical benefit. Overall, the identification and elimination of cysticerci before they degenerate and harden into calcified cysts significantly lowers the risk of developing chronic epilepsy. The range of symptoms caused by NCC, including seizures and epilepsy, extends beyond those experienced from parenchymal and extraparenchymal NCC [19].
Beyond the brain, NCC can also occur and trigger symptoms in other parts of the CNS, such as the spinal cord [15]. Instances of NCC originating in the spine are rare but nonetheless possible [22]. Affected individuals may experience different symptoms depending on where cysticerci are located along the spinal cord [15, 23]. The presence of cysticerci can lead to compression of the spinal cord, which may cause symptoms including muscle weakness and movement problems [23]. In some cases, cysticerci may develop across several parts of the spine, leading to widespread dysfunction [23]. Dysfunction can include anything from general feelings of weakness to total paralysis of the limbs [23, 24]. The vast range of symptoms and respective classifications make NCC a complex disease that can affect many different aspects of a person’s health, contributing to both diagnostic and treatment challenges [15].
Same Parasite, Different Story: Diagnosis and Destruction
Diagnosing any kind of NCC typically requires a combination of screening, medical imaging, and blood tests [6, 25]. If Paul comes into a clinic with possible symptoms of NCC, healthcare professionals will first look at Paul’s medical history and risk factors for clues — such as whether he lives around livestock — that could increase his risk of becoming infected [6, 25]. Healthcare providers may then image his brain using techniques such as magnetic resonance imaging (MRI), which utilizes powerful magnets and radio waves to generate detailed pictures of the brain [6, 25, 26]. If cysticerci are seen or suspected in an MRI or other types of diagnostic images, follow-up blood tests can use antibodies to detect antigens associated with the T. solium tapeworm [27, 28, 29]. A positive blood test for these antigens helps providers confirm Paul’s NCC diagnosis [27, 28, 29]. Following NCC diagnosis, Paul’s treatment plan would depend on where the cysticerci are located, how many there are, and their stage of degeneration [6, 31, 32]. A ‘one size fits all’ treatment plan is therefore ineffective for NCC, but the same general strategies are followed in most cases [6, 31, 32]. First, a healthcare provider might focus on reducing Paul’s symptoms by using several different medications [30, 32]. Some common examples include corticosteroids to lower inflammation and swelling and antiepileptics to reduce the frequency and intensity of seizures [30, 32]. When combined, these medications can target multiple symptoms at once, which can improve overall treatment outcomes [30, 32]. Alongside symptom relief, a provider may simultaneously prescribe drugs that kill Paul’s cysticerci, known as cysticidal drugs [4, 30]. Cysticidal treatments work in a variety of ways — most commonly by slowly interfering with the vital functions of cysticerci until they break apart [30]. Once cysticerci begin to die from cysticidal treatment, their contents, including antigens, leak out and can either generate or intensify a preexisting inflammatory response [30]. Concurrent use of corticosteroids alongside cysticidal drugs can help ease any excessive inflammation [2, 15].
Still, cysticidal drugs are not a viable option for every person, even when combined with anti-inflammatory corticosteroids [30]. Symptoms might not improve at first and could initially worsen for some individuals [30]. Worsening symptoms are especially dangerous in people who already have severe symptoms caused by NCC, such as increased pressure in the skull [30]. Paul has an acute case with a large cysticercus that is causing dangerous, rapidly appearing symptoms that led him to go to the emergency room [1, 33]. The large size of his cysticercus may mean it could take days to shrink using drugs and cause complications from inflammation [33]. With such a severe case, Paul might not survive long enough for this treatment to run its course, and a medical team at a hospital would likely decide that surgical removal is the best option [33]. Because of differing factors and treatments, NCC can be complicated to manage but is considered curable [34]. Still, the disease outlook following treatment depends on the individual case, especially cysticerci location [34].
Early Detection Gets the Worm
Early detection and diagnosis of NCC are particularly challenging in regions where the disease is most common [35]. NCC prevalence is highest in certain parts of Africa, Asia, and South America, typically in countries that lack critical infrastructure [35]. Inadequate sanitation and health education systems are just some examples of weak infrastructure that public health authorities can struggle with [3, 25, 36]. As with many treatable diseases, a shortage of resources can mean many people do not get the proper testing or treatment they need [37]. Likewise, NCC is most likely underestimated and misdiagnosed because the symptoms, such as headaches and seizures, can look very similar to those of other brain disorders [25, 36]. In addition, carriers of the T. solium parasite that could pass eggs on to other people may be asymptomatic, so they are seldom screened [36]. When combined with a general lack of public awareness surrounding NCC, limited screening, and more misdiagnoses contribute to the challenges surrounding the disease [36].
NCC remains a neglected and likely underreported disease, lacking adequate monitoring in many of the countries most deeply impacted [2, 10, 35]. As a result, while increased rates of epilepsy are likely a consequence of increased rates of NCC, large datasets on NCC occurrence can be lacking [2, 10]. Mandatory case reporting could help health authorities more accurately assess how NCC affects communities, both socially and economically [2]. Overall, addressing challenges related to NCC would benefit from a multifaceted approach. Improving sanitation infrastructure — such as increased access to clean water, better waste management, and campaigns to deworm livestock — alongside public education on food safety and disease awareness could significantly reduce the burden of NCC [10]. In addition to enhanced monitoring and infrastructure, prevention strategies are particularly essential. Improving access to rapid diagnostic blood tests that do not require a lab, human vaccine development, and enhanced biological data collection would make a large impact on the field [3]. Investment in affordable, easy-to-use diagnostic tools like immediate-use blood testing kits could improve early detection, particularly in settings with limited resources [3]. Currently, one of the most definitive ways to confirm an infection is by visualizing the parasite in a tissue sample or by MRI [38]. However, this is impractical for widespread use due to the financial burden of specialized equipment and staff [3, 38]. Moreover, blood tests that can detect T. solium in a controlled hospital setting are not sensitive or specific enough to be used widely in the field and are relatively expensive [3]. Research into making blood tests cheaper and more precise would greatly improve disease control efforts [3].
Furthermore, while a vaccine is available for pig cysticercosis, no such vaccine exists for humans [3]. The lack of a human vaccine is partly because developing vaccines for pigs is cheaper, and vaccinating humans has not been considered practical in the past because not enough highly specific target antigens have been discovered for T. solium [3]. A theoretical vaccine would rely on stimulating the immune system by introducing select, very specific antigens associated with T. solium so that the immune system could remember and recognize the actual tapeworm in the case of future exposure [39]. When introduced to these select T. solium antigens, the immune system produces antibodies that match and bind to them [39]. In an active parasitic infection, these antibodies would then be able to neutralize a parasite’s ability to establish in the body [39]. Progress on a human vaccine is slow, but some new specific vaccine targets have recently been discovered, including a protein associated with T. solium reproductive cycles [3, 40]. Adding a T. solium vaccine to childhood vaccination plans could considerably reduce infections in communities where the parasite is most often found [3]. Still, any potential vaccine would face challenges due to cost; even effective vaccines designed for pigs have not been widely used due to financial barriers [3]. Reducing the prevalence of NCC and associated health issues requires further long-term investment [3, 35, 41].
NCC is a significant global public health challenge at the intersection of complex biological and socioeconomic considerations [3]. The complexity of NCC is demonstrated by individual differences in how NCC manifests and unanswered questions about how the disease drives symptoms from mild headaches to fatal neurological complications [1, 6, 30]. Long-term work addressing NCC will require a comprehensive approach that brings clinical practice and policy together [35, 41]. Global financial investment in improved infrastructure, public education, and clinical tools is required [41]. By combining targeted therapies, improved early detection, and preventive strategies, we can work toward reducing and someday eliminating the global impact of NCC and protect communities from an entirely preventable cause of serious neurological symptoms [3].
References
Rizvi, S. A. A., Saleh, A. M., Frimpong, H., Al Mohiy, H. M., Ahmed, J., Edwards, R. D., & Ahmed, S. S. (2016). Neurocysticercosis: A case report and brief review. Asian Pacific Journal of Tropical Medicine, 9(1), 100–102. doi:10.1016/j.apjtm.2015.12.020
Gripper, L. B., & Welburn, S. C. (2017). Neurocysticercosis infection and disease–A Review. Acta Tropica, 166, 218–224. doi:10.1016/j.actatropica.2016.11.015
Hossain, M. S., Shabir, S., & Toye, P. (2023). Insights into the diagnosis, vaccines, and control of Taenia solium, a zoonotic, neglected parasite. Parasites & Vectors 16. doi:10.1186/s13071-023-05989-6
Garcia, H. H, Gonzalez, A. E, & Gilman, R. H. (2020). Taenia solium Cysticercosis and Its Impact in Neurological Disease. Clinical Microbiology Reviews, 33. doi:10.1128/cmr.00085-19
Demis, C., Anteneh, M., & Basith, A. (2015). Tapeworms of poultry in Ethiopia: A review. British Journal of Poultry Sciences, 4(3), 44-52. doi:10.5829/idosi.bjps.2015.4.3.96145
Butala, C., Brook, T. M., Majekodunmi, A. O., & Welburn, S. C. (2021). Neurocysticercosis: Current perspectives on diagnosis and management. Frontiers in Veterinary Science, 8. doi:10.3389/fvets.2021.615703
Li, L., He, W., Fan, X., Liu, M., Luo, B., Yang, F., Jiang, N., Wang, L., & Zhou, B. (2023). Proteomic analysis of Taenia solium cysticercus and adult stages. Frontiers in Veterinary Science, 9(9). doi:10.3389/fvets.2022.934197
Trevisan, C., Mkupasi, E. M., Ngowi, H. A., Forkman, B. J., & Maria V. (2016). Severe seizures in pigs naturally infected with Taenia solium in Tanzania. Veterinary Parasitology, 20, 67-71. doi:10.1016/j.vetpar.2016.02.025.
Carmen-Orozco, Rogger P., Bernal-Teran, Edson G., Chile, Nancy, Condori, Beth J., Cooper, Virginia, Fishbeck, Kayla, Gilman, R. H., Palma, Sandra, Rapport, Laura, Trompeter, Grace, & Verastegui, Manuela. R. (2019). In vitro model of postoncosphere development, and in vivo infection abilities of Taenia solium and Taenia saginata. PLOS Neglected Tropical Diseases, 13(3). doi:10.1371/journal.pntd.0007261
Bustos, J., Gonzales, I., Saavedra, H., Handali, S., & Garcia, H. H. (2021). Neurocysticercosis. A frequent cause of seizures, epilepsy, and other neurological morbidity in most of the world. Journal of the Neurological Sciences, 427, doi:10.1016/J.JNS.2021.117527
Marshall, J. S., Warrington, R., Watson, W., & Kim, H. (2018). An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol, 14(S2). doi:10.1186/s13223-018-0278-1
Gandhi, N. A., Bennett, B. L., Graham, N. M. H., Pirozzi, G., Stahl, N., & Yancopoulos, G. D. (2016). Targeting key proximal drivers of type 2 inflammation in disease. Nature Reviews Drug Discovery, 15, 35-50. doi:10.1038/nrd4624
Prodjinotho, U. F., Lema, J., Lacorcia, M., Schmidt, V., Vejzagic, M., Sikasunge, C., Ngowi, B., Winkler, A. S., & Prazeres da Costa, C. (2020). Host immune responses during Taenia solium neurocysticercosis infection and treatment. PLOS Neglected Tropical Diseases, 14(4), doi:10.1371/journal.pntd.0008005
Garcia, H. H., Rodriguez, S., Friedland, J. S. (2014). Immunology of Taenia solium taeniasis and human cysticercosis. Parasite Immunology 36(8), 388-396. doi: 10.1111/pim.12126
White, A.C., Jr., Coyle, C. M., Rajshekhar, V., Singh, G., Hauser, W. A., Mohanty, A., Garcia, H. H., & Nash, T. E. (2018). Diagnosis and treatment of neurocysticercosis: 2017 clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clinical Infectious Diseases, 66(8), 49-75. doi:10.1093/cid/cix1084
Abanto, J., Blanco, D., Saavedra, H., Gonzales, I., Siu, D., Pretell, E. J., Bustos, J. A., & Garcia, H. H. (2021). Mortality in parenchymal and subarachnoid neurocysticercosis. The American Journal of Tropical Medicine and Hygiene, 105(1), 176-180. doi:10.4269/ajtmh.20-1330
Tadevosyan, A., & Kornbluth, J. (2021). Brain herniation and intracranial hypertension. Neurological Clinics, 39(2), 293-318. doi:10.1016/j.ncl.2021.02.005
Ratcliffe, C., Adan, G., Marson, A., Solomon, T., Saini, J., Sinha, S., & Keller, S. S. (2023). Neurocysticercosis-related seizures: Imaging biomarkers. Seizure: European Journal of Epilepsy, 108, 13-23 doi:10.1016/j.seizure.2023.04.005
Steyn, T. J. S., Awala, A. N., de Lange, A., & Raimondo, J. V. (2022). What causes seizures in neurocysticercosis? Epilepsy Currents, 23(2), 105-112. doi:10.1177/15357597221137418
Schaper, F. L., Nordberg, J., Cohen, A. L., Lin, C., Hsu, J., Horn, A., Ferguson, M. A., Siddiqi, S. H., Drew, W., Soussand, L., Winkler, A. M., Simó, M, Bruna, J., Rheims, S., Guenot, M., Bucci, M., Nummenmaa, L., Staals, J., Colon, A. J., Ackermans, L., Bubrick, E. J., Peters, J. M., Wu, O., Rost, N. S., Grafman, J., Blumenfeld, H., Temel, Y., Rouhl, R. P., Joutsa, J., & Fox, M. D. (2023). Mapping lesion-related epilepsy to a human brain network. JAMA Neurology, 80(9), 891-902. doi:10.1001/jamaneurol.2023.1988
Archibald, L. K., & Quisling R. G. (2013). Central nervous system infections. In: Layon, A., Gabrielli, A., & Friedman, W. (eds.) Textbook of Neurointensive Care, 427–517. Springer. doi:10.1007/978-1-4471-5226-2_22
Pal, A., Biswas, C., Ghosh, T., & Deb, P. (2017). A rare case of recurrence of primary spinal neurocysticercosis mimicking an arachnoid cyst. Asian Journal of Neurosurgery, 12(2), 250-252. doi:10.4103/1793-5482.144176
Barrie, U., Badejo, O., Aoun, S. G., Adeyemo, E., Moler, N., Christian, Z. K., Caruso, J. P., El Ahmadieh, T. Y., Ban, V. S., MacAllister, M. C., Reyes, V. P., Hall, K., Whitworth, L., & Bagley, C. (2020). Systematic review and meta-analysis of management strategies and outcomes in adult spinal neurocysticercosis. World Neurosurgery, 138, 504-511. doi:10.1016/j.wneu.2020.03.093.
Manh, B. H., Dat, T., Hai, V. T., He, D. V., Ha, D. D., Que, N. V., & Duc, N. M. (2023). Spinal cysticercosis: A case report, Radiology Case Reports, 18(9), 3269-3273, doi:10.1016/j.radcr.2023.06.037.
Hurła, M., Pikor, D., Kościelecka, K., Drelichowska, A., Banaszek, N., & Paul, M. (2024). Neurocysticercosis—Diagnostic mystery: Current status for Europe. BioMed, 4(3), 302-313; doi:10.3390/biomed4030024
Grover, V. P. B., Tognarelli, J. M., Crossey, M. M. E., Cox, I. J., Taylor-Robinson, S. D., & McPhail, M. J. W. (2015). Magnetic resonance imaging: Principles and techniques: Lessons for clinicians. Journal of Clinical and Experimental Hepatology, 5(3), 246-255, doi:10.1016/j.jceh.2015.08.001
Tang, N. L., Nash, T. E., Corda, M., Nutman, T. B., & O’Connell E. M. (2023). Triplex ELISA for assessing durability of Taenia solium seropositivity after neurocysticercosis cure. Emerging Infectious Diseases, 29(7),1340-1348. doi:10.3201/eid2907.230364
Arroyo, G., Rodriguez, S., Lescano, A. G., Alroy, K. A., Bustos, J. A, Santivañez, S., Gonzales, I., Saavedra, H., Pretell, E. J., Gonzalez, A. E., Gilman, R. H., Tsang, V. C., & Garcia, H. H. (2018). Antibody banding patterns of the enzyme-linked immunoelectrotransfer blot and brain imaging findings in patients with neurocysticercosis. Clinical Infectious Diseases, 66(2), 282–288. doi:10.1093/cid/cix774
Passaro, A., Jänne, P.A., & Peters, S. (2023). Antibody-drug conjugates in lung cancer: recent advances and implementing strategies. Journal of Clinical Oncology, 41(21). doi:10.1200/JCO.23.00013
Del Brutto, O. H. (2020). Current approaches to cysticidal drug therapy for neurocysticercosis. Expert Review of Anti-Infective Therapy, 18(8), 789–798. doi:10.1080/14787210.2020.1761332
Andrade-Mogrovejo, D. A., Gonzales-Gustavson, E., Ho-Palma, A. C., Prada, J. M., Bonnet, G., Pizzitutti, F., Gomez-Puerta, L. A., Arroyo, G., O’Neal, S. E., Garcia, H. H., Guitian, J., & Gonzalez, A. (2022). Development of a dose-response model for porcine cysticercosis. PLOS One, 17(3). doi:10.1371/journal.pone.0264898
Ramamoorthy, S., & Cidlowski, J.A. (2016). Corticosteroids: Mechanisms of action in health and disease. Rheumatic Disease Clinics of North America, 42(1), 15-31. doi:10.1016/j.rdc.2015.08.002
Hamamoto Filho, P. T., Norcia, L. F., Fleury, A., & Zanini, M. A. (2024). Current role of surgery in the treatment of neurocysticercosis. Pathogens, 13(3). doi:10.3390/pathogens13030218
Takayanagui, O.M., & Marques de Haes, T. (2022). Update on the diagnosis and management of neurocysticercosis. Arquivos de Neuro-Psiquiatria 80(5). doi:10.1590/0004-282X-ANP-2022-S115
Carpio, A., Fleury, A., Romo, M. L., & Abraham, R. (2018). Neurocysticercosis: The good, the bad, and the missing. Expert Review of Neurotherapeutics, 18(4), 289–301. doi:10.1080/14737175.2018.1451328
Rajshekhar, V. (2016). Neurocysticercosis. Diagnostic problems & current therapeutic strategies. Indian Journal of Medical Research, 144(3), 319–326. doi:10.4103/0971-5916.198686
Sharma, S., Zapatero-Rodríguez, J., Estrela, P., & O'Kennedy, R. (2015). Point-of-care diagnostics in low resource settings: Present status and future role of microfluidics. Biosensors, 5(3), 577-601. doi:10.3390/bios5030577
Guzman, C., & Garcia, H. H. (2021). Current diagnostic criteria for neurocysticercosis. Research and Reports in Tropical Medicine, 12, 197–203. doi:10.2147/rrtm.s285393
Pollard, A. J., & Bijker, E. M. A. (2021) Guide to vaccinology: From basic principles to new developments. Nature Reviews Immunology, 21, 83–100. doi:10.1038/s41577-020-00479-7
Stutzer, C., Richards, S. A., Ferriera, M., Baron, S., & Maritz-Olivier, C. (2018). Metazoan parasite vaccines: Present status and future prospects. Frontiers in Cellular and Infection Microbiology, 8. doi:10.3389/fcimb.2018.00067
Carpio, A., Romo, M. L., Parkhouse, R. M. E., Short, B., & Dua, T. (2016). Parasitic diseases of the central nervous system: Lessons for clinicians and policy makers. Expert Review of Neurotherapeutics, 16(4), 404-414. doi:10.1586/14737175.2016.1155454