‘Only Love Can Hurt Like This’: Understanding Broken Heart Syndrome
Chloe Bilger
Illustrations by Elizabeth Catizone
Wrought with grief and arms heavy with his daughter’s slain body, the mad king was so devastated that he died of a broken heart — or so the story goes. In Shakespeare’s tragedy, King Lear, King Lear banishes his daughter Cordelia after she fails to proclaim her familial love for him. However, after a deceitful betrayal by his two other daughters, the King himself is banished and searches for Cordelia in an attempt to reconcile with her. Tragically, he finds his beloved daughter dead. The King’s heart breaks at the sight of her body, and in an instant, he dies. Since the 1600s, dying from a broken heart has been seen throughout the classics as a mythical tragedy, striking down great protagonists for centuries [1]. Yet, despite its cultural prevalence, a ‘broken heart’ has only been recognized as a medical condition for the past 30 years [1]. Today, we can interpret King Lear’s tragic death as a case of Takotsubo Syndrome (TTS), also known as Broken Heart Syndrome, a condition where the heart is thought to be physically damaged by one’s emotional stress [2, 3].
‘Hooked On a Feeling’: Catching Octopuses and Uncovering Heart Conditions
First introduced in Japanese literature in the 1990s, TTS is a unique heart condition often initiated by an intensely stressful event, such as the death of a loved one [3, 4]. Until recently, the origins of TTS were masked by its striking resemblance to a heart attack, as both conditions cause chest pain, shortness of breath, and changes to the heart’s rhythm [5, 6, 7]. As a result of overlapping symptoms, an estimated 7,000 to 14,000 Americans with TTS may be misdiagnosed each year, preventing those individuals from receiving proper care [8, 9]. However, despite significant similarities, the underlying mechanisms that incite a heart attack and TTS are particularly different [10, 11]. Heart attacks are often induced by a build-up of fat, cholesterol, and calcium called plaque, which forms along the walls of the coronary arteries that supply blood and, thus, oxygen to the heart [10, 11]. Over time, the plaque can obstruct blood flow to the heart, making it strenuous for the heart to pump blood and eventually leading to a heart attack [10, 11]. In contrast, during TTS, the heart’s left ventricle — the largest chamber of the heart — spontaneously ‘balloons’ or expands outward, causing the ventricle to be temporarily disfigured [11, 12]. The ballooning of the left ventricle in TTS prevents the heart from adequately pumping blood, leaving the heart temporarily dysfunctional [11, 12]. The shape formed by the expanded left ventricle bears a striking resemblance to a traditional ‘Takotsubo’ pot used to catch octopuses in Japan, giving TTS its name [13]. The leading hypothesis behind the ballooning of the left ventricle in TTS suggests that dysfunction in the nervous system’s response to stress is the underlying cause [11, 12]. As a result, understanding how emotionally traumatic stressors induce TTS may illuminate currently unexplored neural connections between the brain and the heart.
‘Don’t Go Breaking My Heart’: Stress Responses in TTS
The nervous system coordinates a physical response to cope with environmental stressors, like the death of a loved one [14]. The brain activates the sympathetic nervous system (SNS), which is a branch of the nervous system responsible for triggering the fight-or-flight response [15]. The SNS can be best understood as the ‘emergency system’ and, once activated, sounds the alarm by releasing catecholamines, a group of hormones and neurotransmitters that increase the body’s heart rate and blood pressure, to prepare the body for potential dangers [7, 16]. Catecholamines involved in TTS include epinephrine, more commonly known as adrenaline, and its precursor molecule, norepinephrine [7, 16, 17, 18, 19]. Normally, stressful events trigger a stress response characterized by the activation of the SNS and the subsequent production of catecholamines [7, 17]. However, in TTS, there is an overactivation of the SNS, leading the brain to send signals to overproduce epinephrine and norepinephrine [17]. The excess of catecholamines creates a type of chemical toxicity that directly damages the myocardium, the muscular layer of the heart that contains specialized heart cells responsible for generating the heart’s contractions [17, 20]. Catecholamines can also indirectly cause damage by promoting constriction of the coronary arteries [17, 20]. When epinephrine and norepinephrine flood the nervous system, they bind to receptors on the coronary arteries, causing the arteries to constrict and thus reducing blood flow and oxygen to the heart [17, 20]. Additionally, the overproduction of catecholamines in TTS makes the heart contract even more frequently, resulting in an imbalance between how hard the heart is working and how much blood is actually being supplied to the heart [17, 20]. Essentially, the tissues in the heart are not receiving enough oxygen for their workload, which can also lead to damage to the myocardium [17, 20]. The myocardial damage may lead to ‘myocardial stunning’ — a dysfunctional state of the heart characterized by a decrease in the heart’s ability to contract and subsequently pump blood [17, 20]. Myocardial stunning may result in the outward ballooning of the left ventricle, forming the Takotsubo pot-like shape, thus causing heart dysfunction [12, 17, 21]. Therefore, if emotional stress facilitated by catecholamines is powerful enough to induce heart dysfunction, investigating TTS may elucidate the connection between emotional processing and physical health [1, 22, 23].
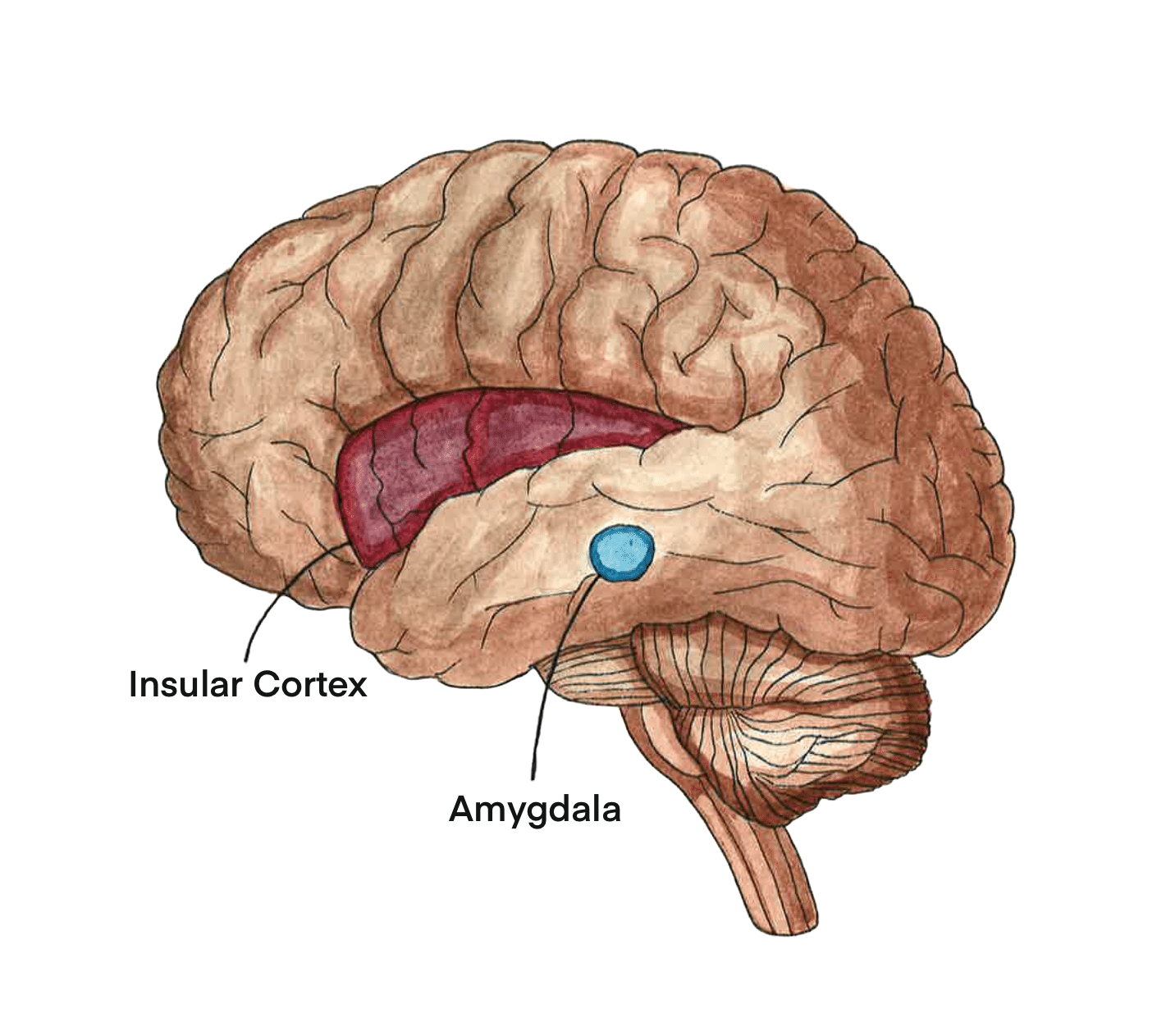
‘Breaking Up Is Hard To Do’: What Happened Between the Insula and Amygdala
When the brain responds to stressful triggers, the SNS is activated by a network of brain regions involved in emotional processing and mediating cardiac responses to emotional stimuli, referred to as the central autonomic network (CAN) [14, 24, 25]. The CAN activates the SNS to carry out stress responses, highlighting a potentially important relationship between the CAN and TTS [14, 24] One important component of the CAN is a brain structure called the insular cortex (IC), which integrates emotional stimuli and stimulates the nervous system to generate a physiological response [14]. The IC is primarily responsible for emotional processing and, as such, helps assign emotional valence or the emotional connotation to an experience or event [14]. Interestingly, studies involving neural lesions — controlled damage to brain tissue — have further illuminated the connection between the IC, excess norepinephrine production, and thus TTS [26, 27]. To determine which neural structures are involved in heart dysfunction, neural lesions were induced in various brain regions, including the IC [26]. Lesions in the IC result in the release of norepinephrine and subsequent heart dysfunction, with more severe lesions correlating with higher release of norepinephrine and more severe dysfunction [16, 24, 26, 27, 28]. The correlation between lesions in the IC, norepinephrine release, and heart dysfunction suggests that dysfunction in brain regions of the CAN may play an important role in TTS [26, 27].
The CAN also includes the amygdala, a structure that is similarly crucial in processing stressful emotions [29]. The amygdala helps assign negative emotional valences to experiences and manages the body’s stress response through the nervous system [25, 29, 30]. Ideally, stress responses are facilitated by communication between the CAN and the SNS, leading to catecholamine production and other physiological responses [24]. However, in TTS, communication between regions in the CAN may be disrupted by alterations in functional connectivity — levels of simultaneous neuronal activation between disparate brain regions — within the CAN [9, 25, 31]. Interestingly, TTS has been linked with both increases and decreases in insular-amygdala functional activity, indicating that deviation in either direction may result in dysfunction [9, 25, 29]. In TTS, alterations in connectivity between brain regions like the IC and amygdala may then instigate the overactivation of the SNS and thus overproduction of catecholamines [9, 17, 24, 25]. Suppose we consider the cause of King Lear’s death to be TTS: the overwhelming stress from the death of his daughter served as the stressful stimulus [14]. King Lear’s CAN works to process the emotional burden and initiates the stress response throughout his body [14]. Consequently, King Lear’s SNS was overactivated, leading to an excessive release of catecholamines, possibly causing the ballooning and left ventricular dysfunction that is characteristic of TTS [17, 32].
Not ‘Somebody We Used to Know’: King Lear in the Modern Age
The tragic death of the titular character in Shakespeare’s King Lear presents a timeless and compelling narrative that may, in fact, have its roots in a real and medically recognized phenomenon: Takotsubo Syndrome (TTS). While King Lear himself seems to be a relic of old literature, his heartbreak remains a real and prevalent issue. Modern science has now uncovered that the emotional distress experienced in moments of extreme grief or stress can have physiological effects, leading to the cardiac dysfunction seen in TTS [17]. The critical intersection of emotion, the brain, and the heart urges a deeper investigation into how emotional stress can manifest itself in profound physiological consequences [2, 3]. The study of TTS offers valuable insight into how the brain processes emotional pain and how this can trigger a cascade of events that ultimately disrupt the heart’s physical functioning [11, 17] Furthermore, altered brain connectivity observed in TTS cases suggests a potential disruption in the brain's emotional regulation systems in response to extreme stress, offering an exciting avenue for future research [9, 25, 29]. Uncovering the mechanisms governing TTS not only deepens our understanding of the syndrome but also encourages a broader exploration of how emotions influence physical health [1, 22, 23]. As scientific knowledge of TTS evolves, it could pave the way for more effective treatments for individuals suffering from both emotional and physical trauma, emphasizing the need for integrated approaches to emotional and cardiovascular health [11]. In Act III, King Lear laments, “Is there any cause in nature that makes these hard hearts?” As it turns out, there is [33].
Reference List
Boyd, B., & Solh, T. (2020). Takotsubo cardiomyopathy. JAAPA, 33(3), 24–29. doi:10.1097/01.jaa.0000654368.35241.fc
Hansen, C., & Phillips, B. (2024a). ‘Wilt break my heart?’ Takotsubo syndrome and Shakespeare’s discourse of heartbreak in Antony and Cleopatra and King Lear. Shakespeare, 1–24. doi:10.1080/17450918.2024.2319124
Wang, X., Pei, J., & Hu, X. (2020). The brain-heart connection in Takotsubo syndrome: The central nervous system, sympathetic nervous system, and catecholamine overload. Cardiology Research and Practice, 2020(1), 1–5. doi:10.1155/2020/4150291
Ghadri, J. R., Wittstein, I. S., Prasad, A., Sharkey, S., Dote, K., Akashi, Y. J., Cammann, V. L., Crea, F., Galiuto, L., Desmet, W., Yoshida, T., Manfredini, R., Eitel, I., Kosuge, M., Nef, H. M., Deshmukh, A., Lerman, A., Bossone, E., Citro, R., Ueyyama, T., Corrado, D., Kurisu, S., Ruschitzka, F., Winchester, D., Lyon, A. R., Omerovic, E., Bax, J. J., Meimoun, P. Tarantini, G., Rihal, C., Hassan, S. Y., Migliore, F., Horowitz, J. D., Shimokawa, H., Lüscher, T. F., & Templin, C. (2018). International expert consensus document on Takotsubo syndrome (part I): Clinical characteristics, diagnostic criteria, and pathophysiology. European Heart Journal, 39(22), 2032–2046. doi:10.1093/eurheartj/ehy076
López Libano, J., Alomar Lladó, L., & Zarraga López, L. (2022). The Takotsubo syndrome: Clinical diagnosis using POCUS. POCUS Journal, 7(1), 137–139. doi:10.24908/pocus.v7i1.15296
Singh, T., Khan, H., Gamble, D. T., Scally, C., Newby, D. E., & Dawson, D. (2022). Takotsubo syndrome: Pathophysiology, emerging concepts, and clinical implications. Circulation, 145(13), 1002–1019. doi:10.1161/circulationaha.121.055854
Frank, N., Herrmann, M. J., Lauer, M., & Förster, C. Y. (2024). Exploratory review of the Takotsubo syndrome and the possible role of the psychosocial stress response and inflammaging. Biomolecules, 14(2), 167. doi:10.3390/biom14020167
Prasad, A., Lerman, A., & Rihal, C. S. (2008). Apical ballooning syndrome (tako-tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. American Heart Journal, 155(3), 408–417. doi:10.1016/j.ahj.2007.11.008
Templin, C., Napp, L. C., & Ghadri, J. R. (2016). Takotsubo syndrome: Underdiagnosed, underestimated, but understood? Journal of the American College of Cardiology, 67(16), 1937–1940. doi:10.1016/j.jacc.2016.03.006
Lu, L., Liu, M., Sun, R., Zheng, Y., & Zhang, P. (2015). Myocardial infarction: Symptoms and treatments. Cell Biochemistry and Biophysics, 72(3), 865–867. doi:10.1007/s12013-015-0553-4
Madias, J. E. (2021). Takotsubo cardiomyopathy: Current treatment. Journal of Clinical Medicine, 10(15), 3440. doi:10.3390/jcm10153440
Citro, R., Bellino, M., Merli, E., Di Vece, D., & Sherrid, M. V. (2023). Obstructive hypertrophic cardiomyopathy and Takotsubo syndrome: How to deal with left ventricular ballooning? Journal of the American Heart Association, 12(21). doi:10.1161/jaha.123.032028
Awad, H. H., McNeal, A. R., & Goyal, H. (2018). Reverse Takotsubo cardiomyopathy: A comprehensive review. Annals of Translational Medicine, 6(23), 460. doi:10.21037/atm.2018.11.08
Lamotte, G., Shouman, K., & Benarroch, E. E. (2021). Stress and central autonomic network. Autonomic Neuroscience, 235, 102870. doi:10.1016/j.autneu.2021.102870
Hashemi, M. M., Gladwin, T. E., de Valk, N. M., Zhang, W., Kaldewaij, R., van Ast, V., Koch, S. B., Klumpers, F., & Roelofs, K. (2019). Neural dynamics of shooting decisions and the switch from freeze to fight. Scientific Reports, 9(1). doi:10.1038/s41598-019-40917-8
Ziemssen, T., & Siepmann, T. (2019b). The investigation of the cardiovascular and sudomotor autonomic nervous system—A review. Frontiers in Neurology, 10. doi:10.3389/fneur.2019.00053
Pelliccia, F., Kaski, J. C., & Camici, P. G. (2017). Pathophysiology of Takotsubo syndrome. American Heart Association Journal, 135(24). doi:10.1161/CIRCULATIONAHA.116.027121
Rotenberg, S., & McGrath, J. J. (2016). Inter-relation between autonomic and HPA axis activity in children and adolescents. Biological Psychology, 117, 16–25. doi:10.1016/j.biopsycho.2016.01.015
Murala, S., & Bollu, P. C. (2022). Norepinephrine. Neurochemistry in Clinical Practice, 165–179. doi:10.1007/978-3-031-07897-2_9
Gherasim, L., & Nistor, R. (2022). Neurogenic stunned myocardium as part of stress cardiomyopathy. Mædica, 17(4), 902–910. doi:10.26574/maedica.2022.17.4.902
Y-Hassan, S. (2014). Acute cardiac sympathetic disruption in the pathogenesis of the Takotsubo syndrome: A systematic review of the literature to date. Cardiovascular Revascularization Medicine, 15(1), 35–42. doi:10.1016/j.carrev.2013.09.008
Amin, H. Z., Amin, L. Z., & Pradipta, A. (2020). Takotsubo cardiomyopathy: A brief review. Journal of Medicine and Life, 13(1), 3–7. doi:10.25122/jml-2018-0067
Xiao, X., & Zhang, Y. (2018). A new perspective on the anterior cingulate cortex and affective pain. Neuroscience & Biobehavioral Reviews, 90, 200–211. doi:10.1016/j.neubiorev.2018.03.022
Brazdil, V., Kala, P., Hudec, M., Poloczek, M., Kanovsky, J., Stipal, R., Jerabek, P., Bocek, O., Pail, M., & Brazdil, M. (2022). The role of central autonomic nervous system dysfunction in Takotsubo syndrome: A systematic review. Clinical Autonomic Research, 32(1), 9–17. doi:10.1007/s10286-021-00844-z
Silva, A. R., Magalhães, R., Arantes, C., Moreira, P. S., Rodrigues, M., Marques, P., Marques, J., Sousa, N., & Pereira, V. H. (2019). Brain functional connectivity is altered in patients with Takotsubo syndrome. Scientific Reports, 9(1). doi:10.1038/s41598-019-40695-3
Osawa, A., Nagai, M., Dote, K., Kato, M., Oda, N., Kunita, E., Kagawa, E., Yamane, A., Kobatake, H., Shiota, H., Ishibashi, N., Takahashi, K., & Förster, C. Y. (2021). A mid‐ventricular variant of Takotsubo syndrome: Was it triggered by insular cortex damage? ESC Heart Failure, 8(4), 3408–3412. doi:10.1002/ehf2.13397
Min, J., Farooq, M. U., Greenberg, E., Aloka, F., Bhatt, A., Kassab, M., Morgan, J. P., & Majid, A. (2010). Cardiac dysfunction after left permanent cerebral focal ischemia: The brain and heart connection. Stroke, 40(7), 2560–2563. doi:10.1161/STROKEAHA.108.536086.
Radfar, A., Abohashem, S., Osborne, M. T., Wang, Y., Dar, T., Hassan, M. Z., Ghoneem, A., Naddaf, N., Patrich, T., Abbasi, T., Zureigat, H., Jaffer, J., Ghazi, P., Scott, J. A., Shin, L. M., Pitman, R. K., Neilan, T. G., Wood, M. J., & Tawakol, A. (2021). Stress-associated neurobiological activity associates with the risk for and timing of subsequent Takotsubo syndrome. European Heart Journal, 42(19), 1898–1908. doi:10.1093/eurheartj/ehab029
Khan, H., Gamble, D. T., Rudd, A., Mezincescu, A. M., Abbas, H., Noman, A., Stewart, A., Horgan, G., Krishnadas, R., Williams, C., Waiter, G. D., & Dawson, D. K. (2023). Structural and functional brain changes in acute Takotsubo syndrome. JACC: Heart Failure, 11(3), 307–317. doi:10.1016/j.jchf.2022.11.001
Šimić, G., Tkalčić, M., Vukić, V., Mulc, D., Španić, E., Šagud, M., Olucha-Bordonau, F. E., Vukšić, M., & R. Hof, P. (2021). Understanding emotions: Origins and roles of the amygdala. Biomolecules, 11(6), 823. doi:10.3390/biom11060823
Mohanty, R., Sethares, W. A., Nair, V. A., & Prabhakaran, V. (2020). Rethinking measures of functional connectivity via feature extraction. Scientific Reports, 10(1), 1298. doi:10.1038/s41598-020-57915-w
Nyman, E., Mattsson, E., & Tornvall, P. (2019). Trigger factors in Takotsubo syndrome – A systematic review of case reports. European Journal of Internal Medicine, 63, 62–68. doi:10.1016/j.ejim.2019.02.017
Shakespeare, W., & Orgel, S. (ed.) (1999). King Lear. Penguin Books.