To Fear or Not to Fear: Exploring Fear Through the Lens of Urbach-Wiethe Disease
By Kristen Carroll
Illustrations by Sarah McDonald
You’re standing in line for a haunted house. As you inch closer to the entrance, anticipation builds and you shudder. Upon reaching the entrance, you try to convince yourself that there’s nothing to fear. After all, if haunted houses were truly scary, wouldn’t people not find them fun? With that thought, you have reassured yourself enough to make it to the front of the line. Once it’s finally your turn to enter the house of horror, your body tenses. Glaring strobe lights force you to squint, and blasting fog machines further cloud your vision. As you swat off cobwebs from your clothing, a shadowed figure starts creeping towards you. Your breathing quickens and your heart begins to pound. Suddenly, an actor in a Ghostface mask jumps out from behind you. You scream and run, wishing you had never entered the house in the first place.
This hypothetical haunted house experience demonstrates the mechanisms and processing of fear. In the haunted house, the appearance of fearful stimuli, like Ghostface, led you to experience physiological changes that caused the well-known ‘fight-or-flight’ response. Ghostface triggered a hormone-release cascade that induced physiological changes typical of fear, such as increased heart rate and rapid breathing [1, 2]. Seeing Ghostface also induced cognitive recognition of fear, meaning that alongside sweaty palms and fast heart rate, you also became aware that you were experiencing fear [3]. While feeling fear may seem like a universal aspect of the human experience, there are, in fact, people who do not experience fear [4]. These individuals have a rare genetic disorder called Urbach-Wiethe Disease (UWD), which can inhibit the ‘fight-or-flight’ fear response [4, 5]. A woman diagnosed with UWD, known as S.M., has been a topic of interest for researchers due to her unusual reactions to typically fearful stimuli [6, 7, 8, 9]. To assess the physiological and behavioral components of fear, S.M. was observed in an experiment where she wandered through a haunted house with what was considered “scary” conditions [8]. Walking through the house, S.M. did not experience physiological changes, such as increased heart rate and rapid breathing, and did not cognitively interpret the house’s stimuli as frightening [8, 9]. This extremely rare disorder is fascinating but can put someone — such as S.M. — into dangerous and avoidable situations [6, 8]. In addition to being a nuanced topic in its own right, UWD and related research can teach us more about the physiological and cognitive responses involving fear [5, 6, 7, 9].
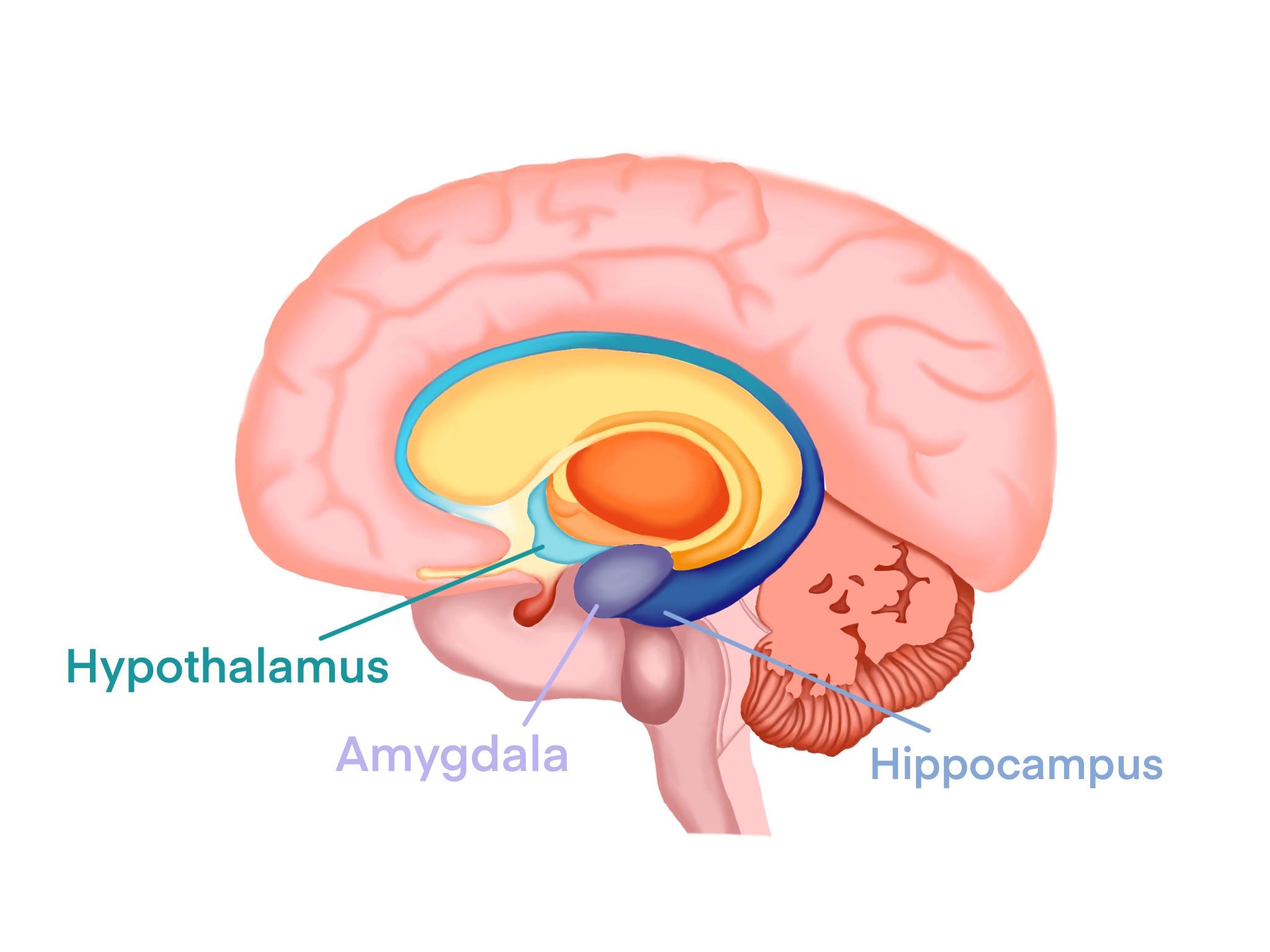
Panic! In the Limbic System
In order to defend the body against potentially harmful stimuli, the limbic system regulates emotional responses that are essential for survival [10]. The key players of the limbic system include the amygdala, the hypothalamus, and the hippocampus; together, these three brain regions process and react to threatening stimuli [10, 11]. Say a child sees a snake and reaches to pick it up. Reacting to her movement, the snake bites her. The painful sensation triggers the child’s amygdala to perform various fear-processing functions, which recognize the snake as a harmful stimulus [7]. The amygdala then sends signals to the hypothalamus and other key brain regions involved in regulating the physiological components of fear [7, 10, 12, 13, 14]. Then, her hypothalamus — a structure that regulates bodily functions by releasing hormones — causes her breathing and heart rate to quicken [10]. Simultaneously, the hippocampus, playing a major role in memory and learning, stores the association between snakes and fear to memory [11]. The next time the child encounters a snake, she is reminded of the painful bite, and experiences the same quickened heart rate and breathing [10, 11]. Reacting to her body’s stress-filled response, she immediately flees [10, 11]. Her limbic system processes her second encounter with a snake in a chain reaction: her hippocampus retrieves the memory of her original encounter, then her amygdala connects that memory to the feeling of fear, causing the hypothalamus to trigger a physiological response [10, 11, 15]. With its rapid response time and ability to learn from past experiences, the limbic system is incredibly important in helping us develop behaviors to avoid subsequent harm [16, 17].
Fear Not, It’s Important!
Despite being an uncomfortable emotion, fear is essential to one’s survival. However, there is debate surrounding how fear is processed in the brain [18, 19]. Much discussion revolves around three predominant theories about how emotions operate: the James-Lange theory, the Cannon-Bard theory, and the Schacter-Singer theory [20]. The James-Lange theory suggests that harmful stimuli trigger a distinct physiological response that we then associate with a specific emotion, such as fear [3, 20]. Thinking back to the snake, imagine the child on a hike, where a snake begins slithering toward her. When she sees the snake, she might begin to sweat, breathe heavily, and feel her heart beating out of her chest [20]. According to the James-Lange theory, the cognitive recognition of these specific physiological changes induces the emotion we know as fear. Alternatively, the Cannon-Bard theory proposes that a harmful stimulus causes us to experience physiological and cognitive reactions simultaneously, leading to the perception of fear. Applying the Cannon-Bard theory, when the child sees the snake, she feels frightened while experiencing an increased heart rate, heavy breathing, and her body shaking. Her physical response alone doesn’t generate her fear; rather, the simultaneous cognitive recognition in conjunction with physiological changes causes her to fear the snake [20]. Finally, the more recent Schacter-Singer theory postulates that we have the same physiological responses to a myriad of stimuli, but the cognitive response to each stimulus is interpreted differently based on the situational context [3, 20, 21, 22, 23]. When the child recognizes the snake, her nervous system prepares her body to either fight or flee the scene [20]. After determining that the situation poses a threat to her safety, her brain labels the physiological response as fear and causes her to flee the snake [20]. However, if someone can’t react to a threatening stimulus or cognitively label it as ‘fear,’ they may behave recklessly when facing the stimulus in the future [24, 25].
Biological Basis of Urbach-Wiethe Disease
Now imagine another scenario where another child doesn’t associate the dangerous snake with fear; she continues towards it and attempts to pick it up [8]! This child may have UWD, a genetic disorder associated with excess calcium build-up in the skin, larynx, and central nervous system, a process called calcification [4, 26, 27]. UWD is caused by mutations in the ECM1 gene, which encodes for a specific type of glycoprotein [4]. Glycoproteins play a variety of roles in the body, such as preventing infection and facilitating cell signaling [28, 29, 30, 31]. In general, the ECM1 gene allows for controlled production of a subset of glycoproteins in the body that primarily contribute to skin integrity [30]. Consequently, a mutation in the ECM1 gene can lead to a build-up of glycoproteins in the skin, larynx, and brain [27]. A surplus of these glycoproteins forms a pale, glassy cartilage known as hyaline [32, 33]. Overproduction of hyaline results in the formation of calcified lesions, a type of abnormal tissue, in the brain and body [4, 34]. Over time, the calcified lesions of the amygdala can cause neural degeneration, significantly reducing fear-processing abilities [4, 5].
Fearless (S.M.’s Version)
Loss of function of the ECM1 gene can lead to the calcification of brain areas including the amygdala, as observed in S.M. [4, 7, 30]. S.M. was born with the ECM1 mutation, which provoked the development of calcified lesions on her amygdala [8]. S.M. recalls experiencing fear as a child but lost the ability to feel fear as the lesions on her amygdala developed over time [35]. Due to the rarity of her condition, S.M. is the subject of many case studies pertaining to UWD and the amygdala’s function in relation to fear [6]. In most cases, S.M. is unresponsive to harmful stimuli that would typically elicit a fearful response and S.M. even has trouble identifying fear in others [7]. When presented with several faces projecting different emotions, S.M. was unable to discern which faces exhibited fearful expressions, thus demonstrating her unresponsiveness to visual expressions of fear [7, 35, 6]. Furthermore, due to her difficulty detecting threats, S.M. reported that she has often found herself in dangerous or even life-threatening situations, such as being held at gunpoint [8]. S.M. claimed that she felt no fear during these traumatic situations; rather, she felt upset and angry [8].
Despite her seeming inability to recognize and experience fear, one study successfully caused S.M. to report the feeling of fear and present with standard physiological responses to a potentially life-threatening stimulus [35]. To study whether someone could feel fear with impaired amygdala function, S.M. and two other participants were given high concentrations of carbon dioxide gas to inhale while under observation [35, 36]. Because inhalation of abnormally high carbon dioxide levels increases breathing and heart rates, and can also induce panic in those inhaling it, this experiment could possibly indicate whether S.M. had any capacity to feel fear [35]. After inhaling an extremely high level of carbon dioxide, S.M. experienced both a panic attack and fear for the first time since childhood. Her experience in the carbon dioxide study was important to our understanding of the amygdala; while the amygdala is critical for triggering the fear response, it did not play a role in the specific type of fear the carbon dioxide triggered in S.M. [35]. During the study, S.M. did not respond to fear that is normally processed through visual or auditory pathways that project to the amygdala [35, 37]. Instead, the carbon dioxide may have been able to trigger S.M.’s fear response by acting on brain structures outside of the impaired amygdala [35, 37]. CO2 inhalation can provoke physiological changes — such as an increase in breathing and heart rate — that are characteristic of experiencing fear [37]. Although S.M. was unable to have a physiological response to visually fearful stimuli — like haunted houses or snakes — she was able to exhibit a fear response when triggered by carbon dioxide inhalation, which appears to bypass the amygdala altogether and seems to be processed through a different pathway [6, 8, 35, 37]. As a result, we now know that some people with UWD can experience intense fear despite a damaged amygdala [35].
Got Fear?
When you find yourself in safe, yet frightening situations —such as haunted house attractions — you may desire a life free from the unpleasant emotion of fear. However, through years of evolution, the fight-or-flight response associated with fear has helped protect us from danger [1, 22]. When cavemen returned home to find a sleeping bear, instantaneous decision-making proved vital for their survival. Although we no longer have to frequently fend off bears, our world is still a perilous one. Due to complications from UWD, individuals like S.M. have found themselves in multiple life-threatening situations because of their inability to connect harmful stimuli with the emotion of fear [6, 8]. As the tissue in S.M.’s amygdala calcifies, it loses its ability to function, leading to changes in the way S.M. experiences stimuli [35, 37]. Since there is still no unified definition of what fear is or how it operates on a universal scale, studying unusual reactions to fearful stimuli can help us better understand the amygdala and other brain regions involved in the fear response pathway. Doing so can lead to new discoveries, such as the pathway that triggers physiological changes and alternative pathways for fear processing [35, 37].
References
Chu, B., Marwaha, K., Sanvictores, T., & Ayers, D. (2022). Physiology, stress reaction. In StatPearls. StatPearls Publishing. PMID:31082164
Scott-Solomon, E., Boehm, E. & Kuruvilla, R. The sympathetic nervous system in development and disease. (2021). Nature Reviews Neuroscience, 22, 685–702. doi:10.1038/s41583-021-00523-y
Dror, O. E. (2016). Deconstructing the “two factors”: The historical origins of the Schachter–Singer theory of emotions. Emotion Review, 9(1), 7–16. doi:10.1177/1754073916639663
Koen, N., Fourie, J., Terburg, D., Stoop, R., Morgan, B., Stein, D. J., & van Honk, J. (2016). Translational neuroscience of basolateral amygdala lesions: Studies of Urbach-Wiethe disease. Journal of Neuroscience Research, 94(6), 504–512. doi:10.1002/jnr.23731
Markowitsch, H. J., Staniloiu, A., & Wahl-Kordon, A. (2023). Urbach-Wiethe disease in a young patient without apparent amygdala calcification. Neuropsychologia, 183, 108505. doi:10.1016/j.neuropsychologia.2023.108505
Barrett, 11. L. F. (2018). Seeing fear: It’s all in the eyes? Trends in Neurosciences, 41(9), 559–563. doi:10.1016/j.tins.2018.06.009
Šimić, G., Tkalčić, M., Vukić, V., Mulc, D., Španić, E., Šagud, M., Olucha-Bordonau, F. E., Vukšić, M., & R. Hof, P. (2021). Understanding emotions: Origins and roles of the amygdala. Biomolecules, 11(6), 823. doi:10.3390/biom11060823
Feinstein, J. S., Adolphs, R., Damasio, A., & Tranel, D. (2011). The human amygdala and the induction and experience of fear. Current Biology, 21(1), 34–38. doi:10.1016/j.cub.2010.11.042
Adolphs, R., Tranel, D., Damasio, H., Damasio, A. R. (1995) Fear and the human amygdala. Journal of Neuroscience, 15(9), 5879-5891. doi:10.1523/JNEUROSCI.15-09-05879.1995
Lee, H., & Kaang, B.-K. (2023). How engram mediates learning, extinction, and relapse. Current Opinion in Neurobiology, 81, 102723. doi:10.1016/j.conb.2023.102723
Rolls, E. T. (2019). The cingulate cortex and limbic systems for action, emotion, and memory. Handbook of Clinical Neurology, 166, 23–37. doi:10.1016/b978-0-444-64196-0.00002-9
Smith, D. M., & Torregrossa, M. M. (2021). Valence encoding in the amygdala influences motivated behavior. Behavioural Brain Research, 411, 113370. doi:10.1016/j.bbr.2021.113370
Herman, J. P., Nawreen, N., Smail, M. A., & Cotella, E. M. (2020). Brain mechanisms of HPA axis regulation: Neurocircuitry and feedback in context Richard Kvetnansky lecture. Stress, 23(6), 617–632. doi:10.1080/10253890.2020.1859475
Barry, T. J., Murray, L., Fearon, P., Moutsiana, C., Johnstone, T., & Halligan, S. L. (2017). Amygdala volume and hypothalamic-pituitary-adrenal axis reactivity to social stress. Psychoneuroendocrinology, 85, 96–99. doi:10.1016/j.psyneuen.2017.07.487
Keifer, O. P., Hurt, R. C., Ressler, K. J., & Marvar, P. J. (2015). The physiology of fear: Reconceptualizing the role of the central amygdala in fear learning. Physiology, 30(5), 389–401. doi:10.1152/physiol.00058.2014
Kim, W. B., & Cho, J.-H. (2020). Encoding of contextual fear memory in hippocampal–amygdala circuit. Nature Communications, 11(1). doi:10.1038/s41467-020-15121-2
Kamali, A., Milosavljevic, S., Gandhi, A., Lano, K. R., Shobeiri, P., Sherbaf, F. G., Sair, H. I., Riascos, R. F., & Hasan, K. M. (2023). The cortico-limbo-thalamo-cortical circuits: An update to the original Papez Circuit of the human limbic system. Brain Topography, 36(3), 371–389. doi:10.1007/s10548-023-00955-y
Mobbs, D., Adolphs, R., Fanselow, M. S., Barrett, L. F., LeDoux, J. E., Ressler, K., & Tye, K. M. (2019). Viewpoints: Approaches to defining and investigating fear. Nature Neuroscience, 22(8), 1205–1216. doi:10.1038/s41593-019-0456-6
Raber, J., Arzy, S., Bertolus, J. B., Depue, B., Haas, H. E., Hofmann, S. G., Kangas, M., Kensinger, E., Lowry, C. A., Marusak, H. A., Minnier, J., Mouly, A.-M., Mühlberger, A., Norrholm, S. D., Peltonen, K., Pinna, G., Rabinak, C., Shiban, Y., Soreq, H., van de Kooij, M. A., Lowe, L., Weingast, L. T., Yamashita, P., & Boutros, S. W. (2019). Current understanding of fear learning and memory in humans and animal models and the value of a linguistic approach for analyzing fear learning and memory in humans. Neuroscience & Biobehavioral Reviews, 105, 136–177. doi:10.1016/j.neubiorev.2019.03.015
Reddy, R. P., Korde, S. P., Kanungo, S., Thamodharan, A., Rajeswaran, J., Bharath, R. D., Upadhya, N., Panda, R., & Rao, S. L. (2014). Neural correlates of emotion: Acquisition versus innate view point. Indian Journal of Psychological Medicine, 36(4), 385–391. doi:10.4103/0253-7176.140720
Reisenzein, R. (2016). The legacy of cognition-arousal theory: Introduction to a special section of Emotion Review. Emotion Review, 9(1), 3–6. doi:10.1177/1754073916662551
Adolphs, R. (2014). The Biology of Fear. Current Biology, 23(2). https://doi.org/10.1016/j.cub.2012.11.055
Schachter, S., & Singer, J. (1962). Cognitive, social, and physiological determinants of emotional state. Psychological Review, 69(5), 379–399. doi:10.1037/h0046234
Beckers, T., Hermans, D., Lange, I., Luyten, L., Scheveneels, S., & Vervliet, B. (2023). Understanding clinical fear and anxiety through the lens of human fear conditioning. Nature Reviews Psychology, 2(4), 233–245. doi:10.1038/s44159-023-00156-1
Cardinale, E. M., Reber, J., O’Connell, K., Turkeltaub, P. E., Tranel, D., Buchanan, T. W., & Marsh, A. A. (2021). Bilateral amygdala damage linked to impaired ability to predict others’ fear but preserved moral judgements about causing others fear. Proceedings of the Royal Society B: Biological Sciences, 288(1943), 20202651. doi:10.1098/rspb.2020.2651
Chatterjee, A., Viswanathan, L., Nagappa, M., & Sinha, S. (2021). Lipoid proteinosis (Urbach-Wiethe disease): A rare genodermatosis with characteristic dermatological and neuroimaging findings. Annals of Indian Academy of Neurology, 24(5), 761. doi:10.4103/aian.aian_1049_20
Parida, J. R., Misra, D. P., & Agarwal, V. (2015). Urbach-Wiethe syndrome. BMJ Case Reports. doi:10.1136/bcr-2015-212443
Ceciliani, F., & Lecchi, C. (2019). The immune functions of α1-acid glycoprotein. Current Protein & Peptide Science, 20(6), 505–524. doi:10.2174/1389203720666190405101138
Banerjee, N., Mukhopadhyay, S. (2016). Viral glycoproteins: Biological role and application in diagnosis. VirusDisease, 27, 1–11. doi:10.1007/s13337-015-0293-5
Li, M., Fischer, J., Safwat, S., Shoman, W., Chazli, Y. E., Alter, S., Has, C., & Abdalla, E. (2022). Lipoid proteinosis: Novel ECM1 pathogenic variants and intrafamilial variability in four unrelated Arab families. Pediatric Dermatology, 40(1), 113–119. doi:10.1111/pde.15105
Schjoldager, K. T., Narimatsu, Y., Joshi, H. J., & Clausen, H. (2020). Global view of human protein glycosylation pathways and functions. Nature Reviews Molecular Cell Biology, 21(12), 729–749. doi:/10.1038/s41580-020-00294-x
Swain, S. K., Sahu, M. C., & Kavita, M. (2017). A comprehensive review on lipoid proteinosis with emphasis on ECM1 gene mutation. Apollo Medicine, 14(2), 105–112. doi:10.1016/j.apme.2017.05.002
Yu, L., Lin, Y.-L., Yan, M., Li, T., Wu, E. Y., Zimmel, K., Qureshi, O., Falck, A., Sherman, K. M., Huggins, S. S., Hurtado, D. O., Suva, L. J., Gaddy, D., Cai, J., Brunauer, R., Dawson, L. A., & Muneoka, K. (2022). Hyaline cartilage differentiation of fibroblasts in regeneration and regenerative medicine. Development, 149(2). doi:10.1242/dev.200249
Augustine, D., Rao, R. S., & Patil, S. (2021). Hyalinization as a histomorphological risk predictor in oral pathological lesions. Journal of Oral Biology and Craniofacial Research, 11(3), 415–422. doi:10.1016/j.jobcr.2021.05.002
Feinstein, J. S., Buzza, C., Hurlemann, R., Follmer, R. L., Dahdaleh, N. S., Coryell, W. H., Welsh, M. J., Tranel, D., & Wemmie, J. A. (2013). Fear and panic in humans with bilateral amygdala damage. Nature Neuroscience, 16(3), 270–272. doi:10.1038/nn.3323
Savulich, G., Hezemans, F. H., van Ghesel Grothe, S., Dafflon, J., Schulten, N., Brühl, A. B., Sahakian, B. J., & Robbins, T. W. (2019). Acute anxiety and autonomic arousal induced by CO2 inhalation impairs prefrontal executive functions in healthy humans. Translational Psychiatry, 9(1). doi:10.1038/s41398-019-0634-z
Khalsa, S. S., Feinstein, J. S., Li, W., Feusner, J. D., Adolphs, R., & Hurlemann, R. (2016). Panic anxiety in humans with bilateral amygdala lesions: Pharmacological induction via cardiorespiratory interoceptive pathways. The Journal of Neuroscience, 36(12), 3559–3566. doi:10.1523/jneurosci.4109-15.2016